Abstract
WRKY45 transcription factor is a central regulator of disease resistance mediated by the salicylic acid (SA) signaling pathway in rice. SA-activated WRKY45 protein induces the accumulation of its own mRNA. However, the mechanism underlying this regulation is still unknown. Here, we report three lines of evidence showing that a mitogen-activated protein kinase (MAPK) cascade is involved in this regulation. An inhibitor of MAPK kinase (MAPKK) suppressed the increase in WRKY45 transcript level in response to SA. Two MAPKs, OsMPK4 and OsMPK6, phosphorylated WRKY45 protein in vitro. The activity of OsMPK6 was rapidly upregulated by SA treatment in rice cells. These results suggest that WRKY45 is regulated by MAPK-dependent phosphorylation in the SA pathway.
The salicylic acid (SA) signaling pathway plays an important role in the defense responses against pathogens in plants. In Arabidopsis, the transcriptional co-activator, NPR1, plays a central role in the SA pathway.Citation1 In rice, the transcription factor, WRKY45 and OsNPR1 play a crucial role in the branched SA pathway.Citation2,Citation3 Arabidopsis NPR1 is degraded by the ubiquitin proteasome system (UPS) and a dual role is proposed for this regulation: prevention of unnecessary defense activation (negative role) and promotion of transcriptional activity upon reception of the SA signal (positive role).Citation4 In rice, UPS-mediated degradation was not observed with OsNPR1, however, it was observed with WRKY45.Citation5 In addition, our previous data suggested a dual role for UPS-mediated degradation of WRKY45 similar to that for Arabidopsis NPR1.Citation5 In Arabidopsis, phosphorylation of NPR1 facilitates its recruitment to UPS, thereby playing a critical role in the positive role.Citation4 In rice, phosphorylated WRKY45 has been detected.Citation5 However, its relevance to the regulation of WRKY45 is unknown. Phosphorylation of WRKY family proteins by mitogen activated protein kinase (MAPK) family proteins is known in Arabidopsis and tobacco.Citation6 Here, we present data suggesting that SA-dependent phosphorylation by MAPK plays a role in the activation of WRKY45, and show that two MAPKs, OsMPK4 and OsMPK6, phosphorylate WRKY45 in vitro.
To test the possible involvement of MAPKs in the activation of WRKY45, we treated cultured rice cells (Oc cells) with U0126, an inhibitor of MAPK kinase (MAPKK) and staurosporine, a protein kinase inhibitor. The SA-activated WRKY45 protein is known to induce the transcripts for WRKY45 itself by autoregulation.Citation5 As expected, WRKY45 transcripts rapidly increased after SA-treatment of the cells in the absence of the inhibitors (). However, U0126 and staurosporine suppressed the transcriptional upregulation in response to SA (), suggesting the involvement of a MAPK(s) in the activation of WRKY45. MAPKs prefer Ser/Thr-Pro signatures as target sites and WRKY45 has three of them at positions 6, 37 and 266. Thus, we examined in vitro the direct phosphorylation of recombinant WRKY45 protein, amino-terminally fused with maltose binding protein (MaBP-WRKY45), by OsMPK4 and OsMPK6, amino-terminally fused with glutathione S-transferase (GST-MPK4 and GST-MPK6). For the in vitro activation of GST-MPKs, we tested several GST-tagged MAPKKs in their active form: OsMKK1, OsMKK3, OsMKK4, OsMKK6 and OsMKK10-2 (GST-MKK1DD, GST-MKK3DD, GST-MKK4DD, GST-MKK6DD and GST-MKK10-2D). Activity assays of phosphorylation of the conventional substrate, myelin basic protein (MyBP), revealed that GST-MPK4 and GST-MPK6 were selectively activated by these MAPKKs (). GST-MPK4 was activated by GST-MKK6DD, and GST-MPK6 by GST-MKK4DD and GST-MKK10-2D. GST-MKK1DD activated both MPKs (). All the activated GST-MPKs, except for the GST-MPK6 activated by GST-MKK1DD, clearly phosphorylated MaBP-WRKY45 (). Thus, we concluded that both OsMPK4 and OsMPK6 directly phosphorylate WRKY45 protein in vitro.
Figure 1. Transcriptional induction of WRKY45 due to autoregulation is suppressed by a MAPKK inhibitor. Oc cells were treated with SA (1 mM) in the presence or absence of a MAPKK inhibitor (1 µM U0126) or a protein kinase inhibitor (10 µM staurosporine). Transcript levels of WRKY45 and ubiquitin (internal standard) were detected by quantitative RT-PCR.
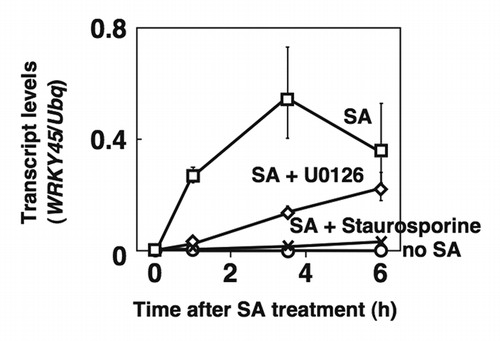
Figure 2. OsMPK4 and OsMPK6 phosphorylate WRKY45 protein in vitro. (A) Purity of recombinant proteins used in the assays. (Left) Coomassie brilliant blue (CBB) staining of GST-fused MKKs and MPKs. (Right) CBB staining and immunoblot assay of MaBP-WRKY45. MaBP-WRKY45 was not purified to a major band in the CBB staining. Anti-WRKY45 antibody was used in the immunoblot assay. (B and C) Phosphorylation assays. Phosphorylation of MyBP (B) and MaBP-WRKY45 (C) as substrates of various combinations of GST-MKKs and GST-MPKs was assayed using [γ-32P] ATP.Citation7
![Figure 2. OsMPK4 and OsMPK6 phosphorylate WRKY45 protein in vitro. (A) Purity of recombinant proteins used in the assays. (Left) Coomassie brilliant blue (CBB) staining of GST-fused MKKs and MPKs. (Right) CBB staining and immunoblot assay of MaBP-WRKY45. MaBP-WRKY45 was not purified to a major band in the CBB staining. Anti-WRKY45 antibody was used in the immunoblot assay. (B and C) Phosphorylation assays. Phosphorylation of MyBP (B) and MaBP-WRKY45 (C) as substrates of various combinations of GST-MKKs and GST-MPKs was assayed using [γ-32P] ATP.Citation7](/cms/asset/043063ad-61d0-455d-a2b1-8c627f60fd98/kpsb_a_10924510_f0002.gif)
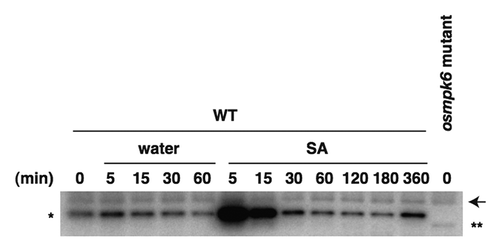
Given that the in vitro assay using MyBP as a substrate can monitor the MAPK activity of phosphorylating WRKY45 (), we performed an in-gel kinase assay using MyBP as a substrate,Citation7 to examine whether SA activates OsMPKs in vivo. Low basal activities were observed in the extract of wild-type (WT) calli and the activity was rapidly and strongly induced by SA (). In contrast, such activity was not detected in the extract of osmpk6 mutant calli. These results indicate that the induced MyBP phosphorylation activity in the SA-treated WT rice callus is due to OsMPK6. The WRKY45 transcripts were induced by SA in the osmpk6 mutant calli to a level comparable with that in WT calli (data not shown). This is presumably due to functional redundancy of OsMPK6 in this regulation, as GST-MPK4, as well as GST-MPK6, phosphorylated MaBP-WRKY45 in vitro (). In addition, MPK3 and MPK6 are known to play redundant roles in Arabidopsis.Citation6,Citation8,Citation9 A faster migrating band was detected in the extract of the osmpk6 mutant (). This activity may be due to OsMPK3 and/or OsMPK4 and compensated for the lack of OsMPK6 in the osmpk6 mutant.
Figure 3. SA treatment rapidly activates OsMPK6 in rice Oc cells. Rice Oc cells were treated with SA or water, and then kinase activities in the cell extracts were assayed by an in-gel assay using MyBP as a substrate.Citation7 The OsMPK6 activity, which is not detected in the osmpk6 mutant, is indicated by *. In the osmpk6 mutant, a faster migrating band due to another kinase activity (**) was detected. An arrow indicates nonspecific bands.
Collectively, our data support a model in which SA activates OsMPK6 and then the activated OsMPK6 directly phosphorylates WRKY45 protein, which is required for its activation. However, it remains unclear how the phosphorylation is involved in the regulation of WRKY45 activity. One possibility is that the SA-dependent phosphorylation modulates the recruitment of WRKY45 protein to UPS-mediated degradation, which is required for its full activation, as reported for Arabidopsis NPR1.Citation4 To test this hypothesis, we replaced serine residues at putative phosphorylation sites of WRKY45 with alanine residues and expressed the mutant form in transgenic rice plants. The estimated molecular weight of the mutant WRKY45 proteins in the transgenic lines was unexpectedly small (data not shown), suggesting they were proteolyzed. However, it is unclear whether the proteolysis is related to UPS degradation. Identification of the WRKY45 phosphorylation sites and their extensive mutation study in transgenic plants would provide more information.
| Abbreviations: | ||
| SA | = | salicylic acid |
| MAPK | = | mitogen-activated protein kinase |
| MAPKK | = | MAPK kinase |
| NPR1 | = | nonexpressor of pathogen- esis-related genes1 |
| OsNPR1 | = | rice (Oryza sativa) NPR1 ortholog |
| UPS | = | ubiquitin proteasome system |
| MaBP | = | maltose binding protein |
| GST | = | glutathione S-transferase |
| MyBP | = | myelin basic protein |
| WT | = | wild type |
| CBB | = | Coomassie brilliant blue |
Acknowledgments
This work was supported by a grant from the Japanese Ministry of Agriculture, Forestry and Fisheries (Genomics for Agricultural Innovation, GMA0001 and PMI0008).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997; 88:57 - 63; http://dx.doi.org/10.1016/S0092-8674(00)81858-9; PMID: 9019406
- Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, et al. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 2007; 19:2064 - 76; http://dx.doi.org/10.1105/tpc.106.046250; PMID: 17601827
- Sugano S, Jiang CJ, Miyazawa S, Masumoto C, Yazawa K, Hayashi N, et al. Role of OsNPR1 in rice defense program as revealed by genome-wide expression analysis. Plant Mol Biol 2010; 74:549 - 62; http://dx.doi.org/10.1007/s11103-010-9695-3; PMID: 20924648
- Spoel SH, Mou Z, Tada Y, Spivey NW, Genschik P, Dong X. Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 2009; 137:860 - 72; http://dx.doi.org/10.1016/j.cell.2009.03.038; PMID: 19490895
- Matsushita A, Inoue H, Goto S, Nakayama A, Sugano S, Hayashi N, et al. The nuclear ubiquitin proteasome degradation affects WRKY45 function in the rice defense program. Plant J 2012; 73:302 - 13; http://dx.doi.org/10.1111/tpj.12035; PMID: 23013464
- Ishihama N, Yoshioka H. Post-translational regulation of WRKY transcription factors in plant immunity. Curr Opin Plant Biol 2012; 15:431 - 7; http://dx.doi.org/10.1016/j.pbi.2012.02.003; PMID: 22425194
- Kishi-Kaboshi M, Okada K, Kurimoto L, Murakami S, Umezawa T, Shibuya N, et al. A rice fungal MAMP-responsive MAPK cascade regulates metabolic flow to antimicrobial metabolite synthesis. Plant J 2010; 63:599 - 612; http://dx.doi.org/10.1111/j.1365-313X.2010.04264.x; PMID: 20525005
- MAPK Group. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 2002; 7:301 - 8; http://dx.doi.org/10.1016/S1360-1385(02)02302-6; PMID: 12119167
- Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 2010; 61:621 - 49; http://dx.doi.org/10.1146/annurev-arplant-042809-112252; PMID: 20441529