Abstract
In the double fertilization of angiosperms, one sperm cell fertilizes an egg cell to produce a zygote, whereas the other sperm cell fertilizes a central cell to give rise to an endosperm. There is little information on gamete membrane dynamics during double fertilization even though the cell surface structure is critical for male and female gamete interactions. In a recent study, we analyzed gamete membrane behavior during double fertilization by live-cell imaging with Arabidopsis gamete membrane marker lines. We observed that the sperm membrane signals occasionally remained at the boundary of the female gametes after gamete fusion. In addition, sperm membrane signals entering the fertilized female gametes were detected. These findings suggested that plasma membrane fusion between male and female gametes occurred with the sperm internal membrane components entering the female gametes, and this was followed by plasmogamy.
Double fertilization is a complex reproduction process specific to angiosperms. Upon pollination, a pollen grain germinates and forms and elongated tube that delivers a pair of sperm cells to the female gametes in an embryo sac. After pollen tube discharge, the two sperm cells come face to face with an egg cell and a central cell. Subsequently, one sperm cell fertilizes the egg cell and the other sperm cell fertilizes the central cell. The egg cell and the sperm cells exist as hemi-protoplasts and protoplasts in the embryo sac, respectively.Citation1,Citation2 Therefore, cell surface structure is critical for male and female gamete interactions in order to regulate double fertilization.
Membrane dynamics during fertilization in living gametes was first investigated in our recent study. A transgenic Arabidopsis expressing pDD45::tRFP-PIP2a (plasma membrane intrinsic protein 2A; PIP2a), which specifically visualized egg cell plasma membrane, was produced and applied as the female gamete membrane marker line for fertilization analyses (hereafter referred to as EC-RFP-PIP).Citation3 To investigate gamete membrane dynamics during double fertilization, a male fluorescent marker line expressing GCS1-GFPCitation4 and HTR10-mRFP, which visualized sperm cell membranes and nuclei, respectively, was applied to the pollination of the female marker line EC-RFP-PIP. GCS1 is also called HAP2 and GCS1-GFP labels both plasma membrane and internal membrane of the sperm cell.Citation4–Citation6 Immediately before the fertilization, bright GFP signals were noted around the sperm cell nuclei, which were closely located at the boundary of the female gametes (). After the initiation of sperm nucleus movement into the female gametes, an apparent GFP signal that diffused at the boundary of the female gametes was observed (). In addition, GFP signals around the sperm cell nuclei at the karyogamy step (characterized by decondensed RFP signals) were rarely observed in the fertilized female gametes (, right arrowhead). To obtain time-lapse images of the gamete fusion process, the semi in vitro double fertilization assay was performed with a male marker line expressing GCS1-GFP and the female marker line EC-RFP-PIP. We observed that the two GFP signals separated and migrated toward opposite directions after being discharged from the pollen tube. The timing of sperm cell movement was considered to be a sign of plasmogamy between male and female gametes in Arabidopsis.Citation7 The signals seemed to gradually diffuse (). In addition, one of the migrating GFP signals merged with the RFP signal.
Figure 1. Sperm cell membrane behavior during double fertilization of Arabidopsis. (A–F) A double male fluorescent marker expressing both HTR10-mRFP and GCS1-GFP was applied to the pollination of the female marker line EC-RFP-PIP. Images before (A–C) and after (D–F) sperm cell movement were captured with an epifluorescence microscope. (A and D) RFP images of the egg cell membrane and the sperm nuclei. Intense RFP signals indicate sperm nuclei labeled with HTR10-mRFP. (B and E) GFP images of sperm cell membrane. Arrowheads indicate GFP remnants. (C and F) Merge of RFP (A and D) and GFP (B and E) images. (G–J) Time-lapse images indicating sperm cell membrane (GCS1-GFP) dynamics with the egg cell membrane marker line EC-RFP-PIP during double fertilization. The images were captured every 5 min with CLSM. Dashed lines indicate egg cell shape based on the RFP signal. Arrowheads indicate GFP signals. The reference time (0 min) corresponds to the time of discharge of sperm cells from the pollen tube.
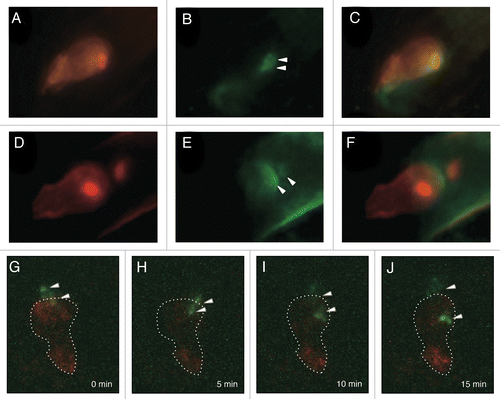
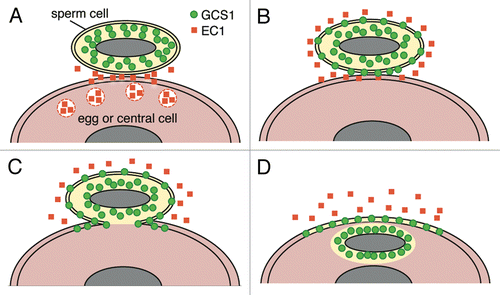
These observations strongly suggested that the GFP signals remaining at the boundary of the female gametes were an indication of plasma membrane fusion between male and female gametes. In addition, GFP signals that entered the female gamete probably reflected the internal membrane of the sperm cell. Therefore, in Arabidopsis double fertilization, plasma membrane fusion occurred, followed by plasmogamy. These processes may be a general phenomenon in angiosperms. A recent study of Arabidopsis demonstrated that endomembrane-associated GCS1 significantly shifted toward the sperm plasma membrane by treatment with synthetic Egg Cell 1 (EC1), a secretory protein expressed in the egg cell.Citation8 In our observation systems, we could not capture such a signal shift in the internal membrane components of the sperm cells. It would be interesting to capture the timing of sperm cell activation by monitoring the shift of GCS1 localization during double fertilization in the future. Taking into account the crosstalk between GCS1 and EC1, the model of Arabidopsis gamete fusion process is shown in .
Figure 2. Putative model of gamete membrane behavior in Arabidopsis double fertilization. (A) A male gamete (sperm cell) contacts a female gamete (egg cell or central cell) after being discharged from a pollen tube. EC1 proteins are secreted by the egg cell. (B) EC1 proteins activate the sperm cell. Re-distribution of GCS1 to the plasma membrane occurs. (C) Plasma membrane fusion of male and female gametes occurs. (D) The sperm cell cytoplasm enters the female gamete (plasmogamy) whereas the sperm cell plasma membrane remains on the surface of the female gamete. The male and female gamete nuclei are pictured as a gray ellipse.
The Arabidopsis GCS1 dynamics observed in our study showed some differences from Chlamydomonas GCS1 dynamics during gamete mating. It was reported that Chlamydomonas GCS1 was rapidly degraded in the zygote immediately after gamete fusion, and that the degradation of GCS1 might have prevented polygamy.Citation9 On the other hand, in Arabidopsis, GCS1-GFP signals remained at the putative gamete membrane fusion site until karyogamy (). This suggests that the persistent GCS1 remnant has no effect on normal double fertilization and that polyspermy blocking in double fertilization is not based on rapid GCS1 degradation, unlike the case of Chlamydomonas.
The distribution of GCS1 is restricted to the mating protrusion in Chlamydomonas, whereas GCS1 molecules are uniformly distributed in the male gametes of plants.Citation4-Citation6,Citation9 EC1 is specific to plant species, although the progression of gamete plasma membrane fusion depends on GCS1 function in both Chlamydomonas and Arabidopsis. It is considered that the signal system of EC1 that causes GCS1 re-distribution has evolved to regulate double fertilization in angiosperms. To facilitate the investigation of gamete fusion systems, other proteins critical to gamete fusion should be identified.
| Abbreviations: | ||
| PIP2a | = | plasma membrane intrinsic protein 2A |
| EC1 | = | Egg Cell 1 |
Acknowledgments
Figure 1 is reprinted with permission from J. Plant Res. Laser scanning microscopy images were acquired with an Olympus FV1000-D at RIKEN BSI-Olympus Collaboration Center. This study was supported by KAKENHI (22112515) to T.I. from JSPS, KAKENHI (22657017) to T.I. from JSPS and KAKENHI (21112008) to T.M. from MEXT.
References
- Nakajima K, Uchiumi T, Okamoto T. Positional relationship between the gamete fusion site and the first division plane in the rice zygote. J Exp Bot 2010; 61:3101 - 5; http://dx.doi.org/10.1093/jxb/erq131; PMID: 20462944
- Okamoto T. Gamete fusion site on the egg cell and autonomous establishment of cell polarity in the zygote. Plant Signal Behav 2010; 5:1464 - 7; http://dx.doi.org/10.4161/psb.5.11.13468; PMID: 21051936
- Igawa T, Yanagawa Y, Miyagishima SY, Mori T. Analysis of gamete membrane dynamics during double fertilization of Arabidopsis. J Plant Res 2012; http://dx.doi.org/10.1007/s10265-012-0528-0; PMID: 23076439
- Mori T, Hirai M, Kuroiwa T, Miyagishima SY. The functional domain of GCS1-based gamete fusion resides in the amino terminus in plant and parasite species. PLoS ONE 2010; 5:e15957; http://dx.doi.org/10.1371/journal.pone.0015957; PMID: 21209845
- Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 2006; 8:64 - 71; http://dx.doi.org/10.1038/ncb1345; PMID: 16378100
- von Besser K, Frank AC, Johnson MA, Preuss D. Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 2006; 133:4761 - 9; http://dx.doi.org/10.1242/dev.02683; PMID: 17079265
- Hamamura Y, Saito C, Awai C, Kurihara D, Miyawaki A, Nakagawa T, et al. Live-cell imaging reveals the dynamics of two sperm cells during double fertilization in Arabidopsis thaliana.. Curr Biol 2011; 21:497 - 502; http://dx.doi.org/10.1016/j.cub.2011.02.013; PMID: 21396821
- Sprunck S, Rademacher S, Vogler F, Gheyselinck J, Grossniklaus U, Dresselhaus T. Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science 2012; 338:1093 - 7; http://dx.doi.org/10.1126/science.1223944; PMID: 23180860
- Liu Y, Misamore MJ, Snell WJ. Membrane fusion triggers rapid degradation of two gamete-specific, fusion-essential proteins in a membrane block to polygamy in Chlamydomonas.. Development 2010; 137:1473 - 81; http://dx.doi.org/10.1242/dev.044743; PMID: 20335357