Abstract
Wheat (Triticum aestivum L.), a staple food crop, is of great commercial importance. Its production is restricted due to multiple environmental stresses. There are indications that the wheat production is consistently limited by terminal heat stress. Previous studies revealed a varied response of different wheat genotypes under heat stress conditions. Here, comparative physiological changes in wheat genotypes viz., DBW-140, Raj-3765, PBW-574, K-0-307 and HS-240 were evaluated under timely and late sown conditions in rabi season. We observed that heat stress dramatically affects chlorophyll content and leaf area index (LAI) in sensitive genotypes whereas proline and malondialdehyde (MDA) content were higher in tolerant genotypes under late sown conditions. Further, the heat susceptibility index (HIS) for 1,000-grain weight, grain weight and grain yield of wheat genotypes viz., HS 240 and K-0-307 was highest as compared with DBW 140, Raj 3765 and PBW 574 genotypes. This finding suggests that wheat genotypes are found to differ in their ability to respond to heat, thereby tolerance, which could be useful as genetic stock to develop wheat tolerant varieties in breeding programs.
Introduction
Wheat is a important cereal second to rice as the main human food crop. In 2011 world production of wheat was 704 million tons.Citation1 Based on latest figures, the overall decrease in world cereal output this year comprises a 5.7 % reduction in wheat production.Citation2 Several environmental constraints specially high temperature and water deficitCitation3-Citation5 are responsible for serious threat in wheat production. The huge yield reduction and quality loss of wheat crop due to terminal heat stress particularly at the time of grain filling is receiving great concern to develop thermotolerance wheat cultivars.Citation6-Citation8 Physiological responses of wheat crop to terminal heat stress have been found to effectively determine genotype resistance or susceptibility.Citation9 The terminal heat stress was at anthesis and grain filling stages accelerate maturity and significantly reduce grain size, weight and yield.Citation10 Plant metabolites in complex biosynthetic pathways are believed to be affected by terminal heat stress.Citation9 It showed the changes in cell membrane structureCitation11 and antixidants including proline accumulationCitation12 and chlorophyll contents and thereby plant senescenceCitation9 which leads to shortening of the period of photosynthetic activity.Citation13 All these impaired physiology of wheat plant under terminal heat stress restrict plant growth and productivity,Citation9 particularly when it occurs during reproductive stages.Citation14 There is urgent need for immediate attention to develop heat tolerance wheat genotypes by combining different approaches. The enhanced membrane thermostability, canopy function, stable green habit, better mobilization rate of food reserve and other technical or physiological parameters might be helpful to overcome yield loss under terminal heat stress.Citation12 In vitro studies of wheat plants in combination with genetic manipulation to develop heat tolerant wheat are of limited success. Therefore, the complex physiological–genetic approach could be useful to acquire heat tolerance in wheat to minimize the farmer’s risk for reduced yield and low quality grain product. Here, Physiological evaluation of wheat genotypes was performed under timely and late sown condition.
Results
Estimation of chlorophyll index (SPAD-values), leaf area index (LAI) and proline content
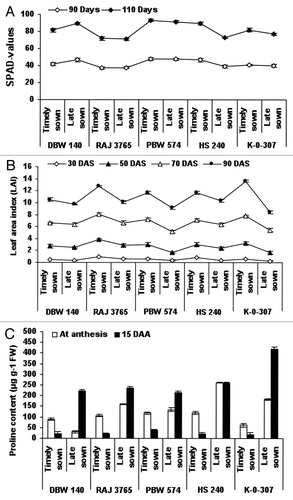
Chlorophyll content (SPAD-values) of wheat genotypes viz., DBW-140, RAJ-3765, PBW-574, K-0-307 and HS-240 under timely and late sown was calculated after 30, 50, 70, 90 and 110 d. It was found that SPAD-values affected under timely and late sown conditions were 41.5, 38.9, 37.08, 37.67, 47.7, 47.59, 46.56, 46.73, 41.03, 40 and 40.15, 34.5, 34.76, 33.33, 45.63, 44, 43.03, 42.52, 4,137 after 90 and 110 d, respectively. Chlorophyll content of PBW-574, K-0-307 and HS-240 genotypes was reduced in late sown condition (38.90, 44.73 and 40.00) as compared with timely sown (34.50, 41.52 and 37.00) after 90 and 110 d. It was little lower in wheat genotype RAJ-3765 after 110 (). Leaf area index (LAI) of tested genotypes was not remarkably affected after 30 d under timely and late sown conditions. Under late sown condition, K-0-307 and HS-240 genotypes showed highest reduction in LAI (0.3, 1.36, 3.78, 3.0 and 0.37, 2.08, 3.94, 3.29) at 30, 70 and 90 d after sowing, respectively (). The proline content (μg g−1 FW) was found to be increased in all the genotypes studied (30.25, 160.5, 135.2, 262.1, 184.0 and 221.68, 236.55, 261.77, 419.35) under late sown condition as compared with that of timely sown condition (89.6, 106.8, 119.6, 62.0 and 21.98, 20.66, 37.0, 20.86, 18.37) at both anthesis and 15 DAS, except DBW-140 (214.67 and 118.6), respectively ().
Figure 1. Estimation of chlorophyll index (SPAD-values), leaf area index (LAI) and proline content in wheat genotypes viz., DBW-140, RAJ-3765, PBW-574, K-0-307 and HS-240 under timely and late sown conditions. Leaf chlorophyll content (SPAD-values) was recorded in the flag leaves, using a self-calibrating SPAD chlorophyll meter (Minolta). Thirty flag leaves per plant were used to calculate the chlorophyll index value that is proportional to the amount of chlorophyll (A). Leaf area index (LAI) was measured with the help of Plant Canopy Analyzer (LAI-2000, LI-COR) (B). Proline content (μg g−1 FW) in wheat genotypes was calculated at anthesis and at 15 DAS (days after anthesis) (C). Vertical lines on top of bars indicate standard error of means.
Evaluation of malondialdehyde (MDA) content, superoxide dismutase (SOD) activity, heat susceptibility index (s) (HIS) and yield associated traits
The amount of malondialdehyde (MDA) (μ mole g−1 FW) produced when polyunsaturated fatty acids in the membrane undergone lipid peroxidation in wheat genotypes were calculated as 13.06, 11.80 and 7.26, 4.23 in HS 240 and K-0-307 genotypes respectively at the time of anthesis in late sown and timely sown conditions. The similar trends were also observed in HS 240 and K-0-307 genotypes 15 DAS, which followed by Raj 3765, DBW 140 and PBW 574 genotypes (). There is no significant increase in superoxide dismutase (SOD) activity (unit mg−1 FW) in genotypes evaluated at anthesis stage under timely and late sown conditions. In contrast, after 15 d of anthesis SOD activity was highest in RAJ-3765 (1.88) whereas in HS-240 and K-0-307 genotypes it was lowest (1.63 and 1.60) under late sown condition (). The heat susceptibility index (HIS) of HS 240 and K-0-307 wheat genotypes was highest (1.33, 1.57, 1.51 and 1.17, 1.34, 1.65), which followed by DBW 140, Raj 3765 and PBW 574 genotypes (0.846, 0.649 and 0.411), (0.780, 0.905 and 0.905) and (0.897, 0.457 abd 0.682) for 1000-grain weight, grain weight and grain yield, respectively (). Yield loss due to terminal heat stress was highest in sensitive genotypes or in the genotypes which are recommended for timely sown condition. The highest yield loss 54.76% was recorded in genotype HS-240 followed by K-0-307 (44.41%) and low yield loss was recorded in DBW-140 followed by Raj 3,765 and PBW-574. The genotype DBW-140 showed highest harvest index in both timely and late sown conditions whereas in HS-240 it was lowest ().
Figure 2. Evaluation of malondialdehyde (MDA) content, superoxide dismutase (SOD) activity and heat susceptibility index (s) (HIS) in wheat genotypes viz., DBW-140, RAJ-3765, PBW-574, K-0-307 and HS-240 under timely and late sown conditions. The extent of lipid peroxidation was evaluated by calculating the concentration of thiobarbituric acid reactive substances as malondialdehyde equivalent using the extinction coefficient (155 mM−1 cm−1) (A). Superoxide dismutase activity was determined by measuring its ability to inhibit the photochemical reduction of nitro-blue tetrazolium (NBT) in the presence of riboflavin in light (B). The heat susceptibility index (s) for yield characters per genotype was calculated (C). Vertical lines on top of bars indicate standard error of means.
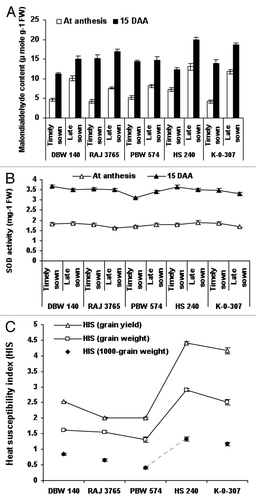
Table 1. Grain yield and harvest index of wheat genotypes under timely and late sown conditions
Discussion
To evaluate wheat genotypes against terminal heat stress, five wheat genotypes were examined for consecutive two years under temperature regime for timely (26.9 ± 1°C) and late sown condition (23.5 ± 1°C). Contents of green plant pigments including carotenoids and their ratios are good indicators of stress detection and tolerance in plants.Citation15 Analysis of variance showed the significant differences between the groups in parameters studied. It has been reported that the terminal heat stress imposes complicate the problems for harvesting higher yield.Citation16 Heat stress declined chlorophyll contents in cool-season cereal species which leads to physiological changes and thereby leaf senescence.Citation17 In this study, chlorophyll content (SPAD value) of heat sensitive genotypes (HS-240 and K-0-307) was highly reduced in late sown condition as opposed to timely sown (). Changes in chlorophyll content are also indicative of tolerant and susceptible nature of genotypes under strong light, high temperature and dry air environmental conditions in timely and late sowing cultivars. Further, it is important to understand that how terminal heat stress particularly impairs chlorophyll biosynthesis. Similarly, leaf area index (LAI) was also affected by genotypes and planting dates.Citation17 In present study, reduction in LAI was high in sensitive genotypes as compared with tolerant genotype. Reduction in LAI was found after 70 and 90 d after sowing variety PBW-574 and K-0-307 showed highest reduction in LAI after 70 d after sowing. Ninety days after sowing maximum reduction in LAI was found in wheat genotype K-0-307 and HS-240 (). Reduction in LAI was fast in late sown condition as compared with timely sown condition. Thus in response to terminal heat stress, the leaf area confines to small to support the required vegetative growth. Genotypic variations in proline accumulation have been observed in many studies and attempts were made to correlate its accumulation with tolerance of plants to stress.Citation18 These findings of the present investigation are similar with that of Gangopadhyay et al.Citation19 who reported that proline regulate the growth under different stresses. Proline was higher in wheat during water stress and its level increased in stress condition.Citation20 This evident correlation between proline accumulation and environmental stress suggests that proline could have a protective function.Citation21 The suitability of proline accumulation under terminal heat stress of timely and late sown wheat genotype is taken into account. In this study, proline was found to be increased in all the genotypes studied under late sown condition except DBW-140 ().
Lipid peroxidation has been found to increase as a prolong exposure to heat.Citation22 High MDA content indicates membrane lipid peroxidation.Citation23 Here, we observed a highest malondialdehyde (MDA) in HS 240 and K-0-307 wheat genotype under late sown conditions (). These findings are in line with the findings of Sairam et al.Citation24 who also reported increase in MDA content in heat sensitive genotypes of wheat. Under optimum temperature conditions, plants maintain a balance between producing and scavenging active oxygen species. Heat stress may disturb this balance and promote lipid peroxidation, either by increasing the production of active oxygen or by decreasing the free radical scavenging ability in cell.Citation25 Heat stress triggers the production of reactive oxygen species (ROS).Citation26 The accumulated of ROS affect physiological process leading to reduce cell function. Therefore, its detoxification by antioxidant systems is important for protecting plant cell against damaged caused by heat stress.Citation27 There is no significant increase in superoxide dismutase (SOD) activity (unit mg−1 FW) in genotypes evaluated at anthesis stage under timely and late sown conditions. In contrast, after 15 d of anthesis SOD activity was highest in RAJ-3765 under late sown condition (). Almeselmani et al.Citation28 who reported that late and very late planting in case of wheat exhibited high SOD activity in late sowing condition. Further, heat stress caused a significant increase in SOD activity both in root and shoot tissue of the hexaploid cultivars.Citation29 In order to determine relative tolerance, heat susceptibility index (HSI) was estimated to characterize wheat genotypes as highly heat tolerant (HSI < 0.50), heat tolerant (HSI: 0.51–0.75), moderately heat tolerant (HSI: 0.76–1.00) and heat susceptible (HSI > 1.00).Citation30 The HSI value for grain yield was maximum in DBW 140 which followed by RAJ-3765 (). The PBW-574 had lowest HSI value (< 1) which explains its heat tolerant potential. In contrast, genotypes HS-240 and K-0-307 had value (> 1) of HSI are susceptible in terms of yield.
The terminal heat stress during growth and development at reproductive stage has been found to affect kernel weight and kernel numberCitation31 and prolong exposure to heat cloud reduce yield and decrease the quality of cereal.Citation32,Citation33 Here, we observed that yield loss due to terminal heat stress under late sown condition was highest in either sensitive genotypes or the genotypes which are recommended for timely sown condition. The highest yield loss 54.76% was recorded in genotype HS-240 followed by K-0-307 (44.41%) and low yield loss was recorded in DBW-140 followed by Raj 3765 and PBW-574. The genotype DBW-140 showed highest harvest index in both timely and late sown conditions and least was in HS-240 (). High temperature stress during post anthesis period of late sown crop might be the reason for its low yield. The findings suggest that the screened genotypes for better yield and high tolerance potential could be used as a genetic stock for further improvement in grain yield during terminal heat stress.
Conclusions
The selection of wheat genotypes with better grain yield and tolerance is the principal aim of wheat production. In this study, terminal heat stress caused significant changes in chlorophyll content, leaf area index grain yield, proline content, lipid peroxidation, grain yield and heat susceptibility index in wheat genotype studied. Significant reductions in chlorophyll content, MDA, LAI, yield and yield components under stress conditions were observed. The protective role of proline content and SOD was evident in tolerant genotypes associated with physiological process and stable yield under terminal heat stress. This study concludes that the wheat genotypes affected by prolonged heat stress are found to differ in their ability to respond, thereby tolerance, which could be useful as genetic stock to develop wheat tolerant varieties in breeding programs.
Materials and Methods
Experimental conditions
The wheat genotypes (Triticum aestivum L) viz., DBW-140, RAJ-3765, PBW-574, K-0-307 and HS-240 were selected for field experiments conducted at Dr N.E. Borlaug Crop Research Centre of GB Pant University Agriculture and Technology, Pantnagar, India in rabi season during 2009–2010. The sowing date (November 20 and December 23) for timely (26.9 ± 1°C) and late sown (23.5 ± 1°C) was similar for 2009- and 2010-field trial. The experiment was conducted in three replicates, in split plot design with seven rows and 23 cm inter row spacing. Weekly maximum and minimum temperature were recorded from meteorological center at Pantnagar throughout the season. Wheat genotype DBW-140, RAJ-3765, PBW-574 were taken as heat tolerant and HS-240 and K-0-307 as heat sensitive.
Evaluation of chlorophyll index and leaf area index
Chlorophyll index was recorded in the flag leaves, using a self-calibrating SPAD chlorophyll meter (Minolta). Thirty flag leaves per plant were used to calculate the chlorophyll index value that is proportional to the amount of chlorophyll. Leaf area index (LAI) was recorded with the help of Plant Canopy Analyzer (LAI-2000, LI-COR). The individual green leaf were measured and expressed as leaf area index (LAI), which represents leaf area per plot area.
Quantification of proline, malondialdehyde (MDA) content and Superoxide dismutase (SOD) activity in wheat genotypes
Wheat leaf segments (500 mg) were homogenized in 10 ml of 3% sulphosalycylic acid followed by centrifugation at 10,000 rpm for 20 min. The supernatant was used for proline estimation. The total proline content was calculated and expressed on fresh weight basis.Citation34
Malondialdehyde (MDA) was measured by the method of Heath and Packer.Citation35 The extent of lipid peroxidation was evaluated by the thiobarbituric acid reaction. Frozen plant tissue was homogenized in 0.1% trichloroacetic acid (1:10, w:v) and centrifuged at 10,000 g for 15 min. One ml of the supernatant was incubated with 4 ml of 0.5% thiobarbituric acid in 20% trichloroacetic acid at 95°C for 30 min in a fume hood and then cooled in ice bath. After centrifugation at 10,000 g for 10 min, the absorbance of the supernatant was read at 532 nm and corrected for the non-specific absorbance recorded at 600 nm. The concentration of thiobarbituric acid reactive substances was calculated as malondialdehyde equivalent using the extinction coefficient (155 mM−1 cm−1).
Superoxide dismutase activity was determined by measuring its ability to inhibit the photochemical reduction of nitro-blue tetrazolium (NBT) in the presence of riboflavin in light.Citation36 One unit of enzyme activity was determined as the amount of the enzyme needed for the inhibition of 50% NBT reduction rate by monitoring absorbance at 560 nm with spectrophotometer.
Growth and yield traits
Physiological traits were recorded for each individual wheat genotype for two consecutive years. The yield characters like grain yield, 1,000 grain weight and grain weight per spike were studied. The heat susceptibility index (s) for yield characters per genotype was calculated by method as described by Fischer and Maurer (1978)Citation37 with the following formula: SI = (1 − Xh/X)/(1 − Yh/Y),where Xh and X are the phenotypic means for each genotype under heat stressed and control conditions, respectively, and Yh and Y are the phenotypic means for all genotypes under heat stressed and control conditions, respectively.
Statistical analysis
Results were analyzed by one-way ANOVA to identify significant differences between the groups and their significance levels (p < 0.05) were determined.
Acknowledgments
Authors are thankful to Director Research, G. B. Pant University of Agriculture and Technology, Pantnagar-263 145 (Uttaranchal, India) for providing research facilities and to Dr Bharti Garg for critical reading of the manuscript. Work on plant stress tolerance in NT’s laboratory is partially supported by Department of Science and Technology (DST) and Department of Biotechnology (DBT), Government of India.(Citation19 Authors note: Please provide in-text reference for ref 19)
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- FAO. Top production world total. 2011. http://faostat3.fao.org/home/index.html#DOWNLOAD
- FAO. FAO Cereal Supply and Demand Brief. 2012. http://www.fao.org/worldfoodsit uation/wfs-home/csdb/en/
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C(3) plants. Plant Biol (Stuttg) 2004; 6:269 - 79; http://dx.doi.org/10.1055/s-2004-820867; PMID: 15143435
- Prasad PVV, Staggenborg SA, Ristic Z. Impacts of drought and/or heat stress on physiological, developmental, growth and yield processes of crop plants. In ‘Response of crops to limited water: understanding and modeling water stress effects on plant growth processes. Advances in Agricultural Systems Modeling Series 1’. (Eds LR Ahuja, VR Reddy, SA Saseendran, Q Yu), 2009; pp. 301–355. (ASA, CSSA, SSSA: Madison, WI, USA)
- Pradhan GP, Prasad PVV, Fritz AK, Kirkham MB, Gill BS. Response of Aegilops species to drought stress during reproductive stages of development. Funct Plant Biol 2012; 39:51 - 9; http://dx.doi.org/10.1071/FP11171
- Rane J, Shoran J, Nagarajan S. Heat stress environments and impact on wheat productivity in India:Guestimate of losses. Indian Wheat News Letter 2000; 6:5 - 6
- Lane A, Jarvis A.. Changes in Climate will modify the Geography of Crop Suitability: Agricultural Biodiversity can help with Adaptation. eJournal 2007; 4:1 - 12
- Kosina P, Reynolds MP, Dixon J, Joshi A. Stakeholder perception of wheat production constraints, capacity building needs, and research partnerships in developing countries. Euphytica 2007; 157:475 - 83; http://dx.doi.org/10.1007/s10681-007-9529-9
- Almeselmani M, Deshmukh PS, Chinnusamy V. Effect of prolong high temperature stress on respiration, photosynthesis and gene expression in wheat (Triticum aestivum L.) varieties differing in their thermotolerance. Plant Stress 2012; 6:25 - 32
- Mohammadi M. Effects of kernel weight and source-limitation on wheat grain yield under heat stress. Afr J Biotechnol 2012; 11:2931 - 7
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot 2007; 61:199 - 223; http://dx.doi.org/10.1016/j.envexpbot.2007.05.011
- Kumar RR, Goswami S, Sharma SK, Singh K, Gadpayle KA, Kumar N, et al. Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzyme, osmolyte, H2O2 and transcript of heat shock protein. Int J Plant Physiol Biochem 2012; 4:83 - 91
- Tewari AK, Tripathy BC. Temperature-Stress-Induced Impairment of Chlorophyll Biosynthetic Reactions in Cucumber and Wheat. Plant Physiol 2012; 117:3 851 - 858
- Hays DB, Do JH, Mason RE, Morgan G, Finlayson SE. Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci 2007; 172:1113 - 23; http://dx.doi.org/10.1016/j.plantsci.2007.03.004
- Zhang X, Wollenweber B, Jiang D, Liu F, Zhao J. Water deficits and heat shock effects on photosynthesis of a transgenic Arabidopsis thaliana constitutively expressing ABP9, a bZIP transcription factor. J Exp Bot 2008; 59:839 - 48; http://dx.doi.org/10.1093/jxb/erm364; PMID: 18272919
- Mahmood S, Wahid A, Javed F, Basra SMA. Heat stress effects on forage quality characteristics of maize (Zea mays) cultivars. International Journal of Agriculture and Biology 2010; 12:701 - 6
- Almeselmani M, Abdullah F, Hareri F, Naaesan M, Ammar MA, Kanbar OZ, et al. Effect of drought on different physiological characters and yield component in different Syrian durum wheat varieties. J Agric Sci 2011; 3:127 - 33
- Ahmed JU, Hasan MA. Evaluation of seedling proline content of wheat genotypes in relation to heat tolerance. Bangladesh J Bot 2011; 40:17 - 22; http://dx.doi.org/10.3329/bjb.v40i1.7991
- Gangopadhyay G, Basu S, Mukherjee BB, Gupta S. Effect of salt and osmotic Shocks on unadapted and adapted callus lines of tobacco. Plant Cell Tissue Organ Cult 1997; 49:45 - 52; http://dx.doi.org/10.1023/A:1005860718585
- Tatar O, Gevrek MN. Influence of water stress on proline accumulation, lipid peroxidation and water content of wheat. Asian J Plant Sci 2008; 7:409 - 12; http://dx.doi.org/10.3923/ajps.2008.409.412
- Handa S, Handa AK, Hasegawa PM, Bressan RA. Proline accumulation and the adaptation of cultured plant cells to water stress. Plant Physiol 1986; 80:938 - 45; http://dx.doi.org/10.1104/pp.80.4.938; PMID: 16664745
- Soliman WS, Fujimori M, Tase K, Sugiyama S. Oxidative stress and physiological damage under prolonged heat stress in C3 grass Lolium perenne.. Grassland Sci 2011; 57:101 - 6; http://dx.doi.org/10.1111/j.1744-697X.2011.00214.x
- Okuda T, Matsuda Y, Yamanaka A, Sagisaka S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol 1991; 97:1265 - 7; http://dx.doi.org/10.1104/pp.97.3.1265; PMID: 16668520
- Sairam RK, Srivastava GC, Saxena DC. Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biol Plant 2000; 43:245 - 51; http://dx.doi.org/10.1023/A:1002756311146
- Bowler C, Van Montagyu M, Inze D. Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 1992; 43:83 - 116; http://dx.doi.org/10.1146/annurev.pp.43.060192.000503
- Almeselmani M, Deshmukh PS, Sairam RK. High temperature stress tolerance in wheat genotypes: role of antioxidant defence enzymes. Acta Agron Hungar 2009; 57:1 - 14; http://dx.doi.org/10.1556/AAgr.57.2009.1.1
- Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant 2006; 126:45 - 51; http://dx.doi.org/10.1111/j.0031-9317.2005.00582.x
- Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci 2006; 171:382 - 8; http://dx.doi.org/10.1016/j.plantsci.2006.04.009; PMID: 22980208
- Eyidoğan F, Öz MT. Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol Plant 2007; 29:485 - 93; http://dx.doi.org/10.1007/s11738-007-0059-9
- Singh K, Sharma SN, Sharma Y. Effect of high temperature on yield attributing traits in bread wheat. Bangladesh. J Agric Res 2001; 36:415 - 26
- Shefazeadeh MK, Mohammadi M, Karimizadeh R, Mohammadinia G. Tolerance study on bread wheat genotypes under wheat stress. Ann Biol Res 2012; 3:4786 - 9
- Mohammadi M, Karimizadeh R, Shefazeadeh MK. Grain yield performance of bread wheat germplasm under heat and drought field conditions. Ann Biol Res 2012; 3:3149 - 55
- Hakim1 MA, Hossain A, Jaime A, Silva TD, Zvolinsky VP, Khan MM. Yield, Protein and Starch Content of Twenty Wheat (Triticum aestivum L.) Genotypes Exposed to High Temperature under Late Sowing Conditions. J Sci Res 2012; 4:477 - 89
- Bates LS, Waldron RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil 1973; 39:205 - 7; http://dx.doi.org/10.1007/BF00018060
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 1968; 125:189 - 98; http://dx.doi.org/10.1016/0003-9861(68)90654-1; PMID: 5655425
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 1977; 59:309 - 14; http://dx.doi.org/10.1104/pp.59.2.309; PMID: 16659839
- Fisher RA, Maurer R. Drought resistance in wheat cultivars I Grain yield response. J Agric Res 1978; 29:898 - 907