Abstract
In Saccharomyces cerevisia, the key gluconeogenic enzyme fructose-1,6-bisphosphatase is secreted into the periplasm during prolonged glucose starvation and is internalized into Vid/endosomes following glucose re-feeding. Fructose-1,6-bisphosphatase does not contain signal sequences required for the classical secretory and endocytic pathways. Hence, the secretion and internalization are mediated via the non-classical pathways.
Gluconeogenic enzymes are induced when Saccharomyces cerevisiae are grown in media containing low glucose. However, when glucose is added to glucose-starved cells, these enzymes are inactivated and degraded. These enzymes include fructose-1,6-bisphosphatase (FBPase), isocitrate lyase (Icl1p), phosphoenolpyruvate carboxykinase (Pck1p) and malate dehydrogenase (MDH2). Inactivation and degradation of gluconeogenic enzymes during glucose re-feeding prevents energy futile cycles that are harmful to cells. FBPase is degraded either in the vacuole or in the proteasome depending on the length of glucose starvation. For the vacuole-dependent pathway, several intermediate compartments are utilized. Vid (vacuole import and degradation) vesicles are small vesicles, whereas Vid/endosomes contain clusters of Vid vesicles. Recent evidence indicates that FBPase is secreted into the periplasm during glucose starvation. Following glucose re-feeding, FBPase is internalized into Vid/endosomes in the cytoplasm. FBPase internalization is dependent on the ARC18 (Arp2/3 complex subunit) and SLA1 (Synthetic Lethal with ABP1) genes involved in actin polymerization/endocytosis. FBPase internalization also requires the VPS34 gene encoding PI3K. Using these unconventional pathways, secreted FBPase is retrieved into the cytoplasm and subsequently degraded in the vacuole.
Autophagy and Human Diseases
Autophagy is a process by which proteins or organelles are degraded in the lysosome /vacuole. Multiple autophagic pathways have been identified.Citation1-Citation4 The best example is the non-selective macroautophagic pathway, which is induced when cells are starved of nutrients.Citation1,Citation2,Citation5 This pathway recycles amino acids for reuse and is important for survival during starvation. In addition, autophagy is critical for a number of biological processes such as extension of life span, developmental regulation and defense against the invasion of pathogens.Citation2,Citation6-Citation10 Altered autophagy is associated with many pathological conditions including aging, cancer, neuromuscular degeneration and neurodegeneration.Citation8,Citation11-Citation13 In animal models of neurodegeneration, rapamycin which induces autophagy reduced large protein aggregates and improved the performance of affected animals.Citation14,Citation15 Therefore, induced autophagy has the potential to treat patients with aggregates-prone diseases such as Parkinson disease, Huntington’s disease, or Alzheimer disease.
Catabolite Inactivation
A novel autophagic pathway that degrades gluconeogenic enzymes during glucose re-feeding has been studied in Saccharomyces cerevisiae. Gluconeogenic enzymes fructose-1,6-bisphosphatase (FBPase), isocitrate lyase (Icl1p), phosphoenolpyruvate carboxykinase (Pck1p) and malate dehydrogenase (MDH2) are induced when cells are grown in media containing low glucose.Citation16-Citation18 However, following a transfer of glucose-starved cells to media containing high glucose, these enzymes are inactivated and degraded.Citation17-Citation24 This inactivation was called “catabolite inactivation” by Dr H. Holzer more than 20 years ago.Citation18 However, the mechanisms responsible for catabolite inactivation have not been completely elucidated. In addition to gluconeogenic enzymes, catabolite inactivation has also been described for mitochondrial enzymes such as the F1 subunit of the ATPase,Citation25 Ach1p involved in acetate metabolism,Citation26 the galactose,Citation27,Citation28 the maltoseCitation29,Citation30 and the high-affinity glucose transporters.Citation18 Thus, catabolite inactivation applies to multiple enzymes involving different metabolic pathways.
The Site of Degradation
Fructose-1,6-bisphosphatase is the key gluconeogenic enzyme and has been used extensively to study the mechanisms for glucose-induced inactivation and degradation.Citation20,Citation21,Citation31-Citation36 This protein has been reported to be degraded either in the vacuoleCitation20,Citation21,Citation37-Citation45 or in the proteasome.Citation31,Citation36 Interestingly, the site of degradation of FBPase varies depending on the length of glucose starvation.Citation32 FBPase is degraded in the proteasome when glucose is added to cells that are starved for 1 d. In contrast, FBPase is degraded in the vacuole when glucose is added to cells that are starved for 3 d. Malate dehydrogenase (MDH2) is another gluconeogenic enzyme that is degraded in the proteasome upon addition of glucose to 1 d-starved cells. This protein is degraded in the vacuole following a transfer of 3 d-starved cells to glucose.Citation32 Likewise, two other gluconeogenic enzymes Pck1p and Icl1p are targeted and then degraded in the vacuole when prolonged-starved cells are replenished with glucose.Citation40 Trafficking of gluconeogenic enzymes to the vacuole in response to glucose has been demonstrated using indirect immunofluorescence microscopy, immuno-transmission electron microscopy (immuno-TEM) and fluorescence microscopy with GFP targeted cargo proteins.Citation20,Citation21,Citation32,Citation40 Targeting of FBPase to the vacuole has also been reconstituted in vitro using semi-permeabilized cells.Citation34
The Vacuole Import and Degradation Pathway
A number of VID (vacuole import and degradation) genes have been identified as being required for the degradation of FBPase in the vacuole.Citation45 Homologs of these VID genes are also found in mice and human, suggesting that VID genes are evolutionarily conserved. The degradation of FBPase, MDH2, Pck1p and Icl1p was retarded in cells lacking the VID24 gene,Citation40 indicating that the Vid pathway mediates the degradation of these proteins in the vacuole. The fact that multiple gluconeogenic enzymes are degraded in the vacuole via the Vid pathway highlights the importance of this pathway. Furthermore, the Vid pathway is a selective degradation pathway. Cargo proteins are degraded when they are no longer needed in new environments. This is different from the starvation-induced autophagic pathway that degrades proteins non-selectively. GID (glucose induced degradation) genes were isolated as being required for the degradation of FBPase in the proteasome.Citation36 Interestingly, many of these GID genes are also involved in vacuole-dependent degradation of FBPase in response to glucose addition.Citation32
For the Vid pathway, FBPase is associated with intermediate compartments prior to being delivered to the vacuole. Vid vesicles are small vesicles and have smooth surfaces.Citation46 These vesicles were detected in glucose-starved cells, suggesting that they are formed prior to the addition of glucose. Vid24p is a peripheral protein that resides on Vid vesicles.Citation44,Citation47 COPI coatomer proteins are also present on Vid vesicles and are required to recruit Vid24p to these vesicles.Citation44 COPI coatomer proteins are involved in multiple trafficking pathways in mammalian cells and in yeast. For example, COPI proteins are required for retrograde transport from the Golgi to the ER. Furthermore, these proteins are localized to endosomes and play important roles in endosomal sorting.Citation48-Citation52 In the absence of the UBC1 (ubiquitin conjugating enzyme 1) gene, levels of Vid24p were reduced in the Vid vesicle enriched fraction, suggesting that the UBC1 gene is involved in the formation of Vid vesicles.Citation33 The import of FBPase into Vid vesicles has been reconstituted in vitro. The sequestration of FBPase requires the heat shock protein Ssa2p, cyclophilin A and Vid22p.Citation38,Citation42,Citation43 Recent evidence indicates that Vid30p is also distributed to Vid vesicles and forms a large protein complex with Vid24p and Sec28p.Citation53 Moreover, the type I phosphatase Reg1p-Glc1pCitation54 and the vacuole ATPaseCitation55 play important roles in the Vid pathway.
FBPase is Localized to Endosomes Following Glucose Addition
Vid vesicles exist in at least two forms. Individual Vid vesicles are 30–50 nm in diameter.Citation46 Vid vesicles can also aggregate to form Vid/endosomes that contain the endosomal protein Pep12p, the Vid-vesicle protein Vid24p and the cargo protein FBPase.Citation39 Vid/endosomes have been purified and examined at the ultra-structural level.Citation39 FBPase and Vid24p were in small compartments inside Vid/endosomes. Vid24p was also present at multiple locations on the surface of Vid/endosomes.Citation39 It is difficult to assess whether or not a layer of common membranes surrounds Vid/endosomes, as such membranes may not be preserved during fixation and processing for negative staining and TEM. The VPH1 gene is required for the Vid pathway at a late step. As such, FBPase accumulated in late endosomes in cells lacking this gene. In the Δvph1 mutant, FBPase-GFP was inside endosomes that were surrounded by a thin layer of FM4-64, suggesting that common membranes are present in these structures.Citation39 FM 4-64 (FM) is a red fluorescence dye that is internalized and subsequently targeted to endosomes and then to the vacuole.Citation56 As such, this dye has been used to label compartments in the endocytic pathway. In addition to FBPase, Vid vesicle-proteins Vid24p and Sec28p also co-localize with FM-containing endosomes upon a transfer of glucose-starved cells to glucose.Citation44
When the distribution of FBPase was examined at the ultra-structural level, FBPase was in areas near the plasma membrane following glucose re-feeding for 15 min.Citation39 In yeast, actin polymerization is needed for scission of endocytic vesicles and generation of force to propel endocytic vesicels.Citation57-Citation60 Interestingly, FBPase and MDH2 associated with actin patches transiently and they dissociated later.Citation39 Vid-vesicle proteins such as Vid24p and Sec28p also associated with actin patches initially. However, less co-localization was observed at later time points.Citation39 The utilization of the endocytic pathway enables cells to remove molecules from the extracellular and intracellular spaces simultaneously. This may provide an efficient way for cells to clear unwanted proteins and to adapt quickly to the new environments.
Anterograde and Retrograde Trafficking to and from the Vacuole
Co-localization of cargo proteins and Vid vesicle proteins such as Vid24p, Sec28p and Vid30p with endosomes suggests that these proteins are delivered to endosomes and to the vacuole via the anterograde trafficking pathway. However, cargo proteins are degraded in the vacuole, whereas Vid vesicle proteins are not degraded in the vacuole. Thus, a retrograde transport pathway should be used to retrieve Vid vesicle proteins from the vacuole. Without retrograde transport, these molecules would be trapped in the vacuole.
To study retrograde transport, the vacuole was pre-labeled with FM4-64 for 16 h in glucose-starved wild-type cells that expressed GFP tagged proteins. Cells were then re-fed with glucose and examined for the distribution of GFP proteins in retrograde transport vesicles that budded from the vacuole. In these studies, Sec28p was detected in vesicles that formed from the vacuolar membrane.Citation40,Citation44 Co-localization of Sec28p in retrograde vesicles requires the UBC1 and the RET2 genes (encodes the δ subunit of COPI coatomers).Citation71 The Tor1 complex (TORC1) plays an essential role in the Vid pathway. Interestingly, the TORC1 subunits Tor1p and Tco89p were also found in retrograde transport vesicles.Citation40 In addition to having a role in retrieving proteins from the vacuole, retrograde transport is also critical to maintain the size of the vacuole. Without retrograde transport, the vacuole membrane would expand. As such, retrograde transport is as important as anterograde transport.
VID30 Plays a Critical Role in the Association of Vid vesicles with Actin Patches
VID30 was originally identified via a transposon library screening for mutants defective in the Vid pathway.Citation53 Vid30p is constitutively expressed and localized to Vid vesicles.Citation53 This protein is associated with actin patches during glucose starvation and less association was observed at later time points. In the absence of the VID30 gene, Vid24p and FBPase did not co-localize with actin patches, suggesting that VID30 is required for the association of Vid vesicles with these patches.
Vid30p contains a lissencephaly type 1-like homology domain (LisH). Mutations in the LIS1 gene cause Miller-Dieker lissencephaly disorder and early death. Vid30p also contains a C-terminal to the LisH domain (CTLH) involved in microtubule dynamics.Citation61-Citation63 These domains are important for Vid30p interaction with Vid24p and Sec28p. In the absence of these domains, FBPase accumulated in punctate structures. In contrast, in the complete absence of VID30, FBPase showed diffused distribution. Because FBPase displayed different distribution patterns, the LisH and CTLH domains are likely to be involved in a late step in the FBPase degradation pathway.Citation53
FBPase is Secreted in Glucose-Starved Cells and Internalized in Glucose Re-fed Cells
The finding that FBPase was distributed to the FM-containing endosomes in glucose re-fed cells raised the possibility that FBPase itself is secreted into the periplasm prior to glucose addition. To investigate this, FBPase distribution was examined at the ultra-structural level.Citation64 When wild-type cells were grown in media containing high glucose, FBPase levels were low. In cells that were starved for 3 d, FBPase was induced and a high percentage of FBPase was in the periplasm. Thus, FBPase is secreted into the periplasm during prolonged starvation. Following the addition of glucose to 3 d-starved cells for 15 min, FBPase appeared in Vid/endosomes in the cytoplasm. In cells that were starved for 3 d and then re-fed with glucose for 120 min, total amounts of FBPase decreased, indicating that most of the FBPase is degraded by this time point.
The appearance of FBPase in the extracellular fraction was further demonstrated using a protocol that extracts extracellular proteins from whole cells. In these studies, wild-type cells were starved, re-fed with glucose and extracellular proteins were extracted. Proteins were then separated into the extracellular and intracellular fractions and the distribution of proteins in these fractions was determined. This protocol was used to detect the secretion of mammalian galectin-1 expressed in Saccharomyces cerevisiae.Citation65 Similar protocols have been employed to study cell-wall associated proteins in C. albicans.Citation66
This protocol was utilized to demonstrate that molecules involved in the Vid pathway were distributed mostly in the intracellular fraction.Citation64 Lst8p and Tor1p are subunits of the Tor1p complex and were mainly in the intracellular fraction. Likewise, the majority of the Sec28p, Vid24p, Vid30p and Vps34p were in the intracellular fraction. By contrast, FBPase was in both intracellular and extracellular fractions. Interestingly, the appearance of FBPase in the extracellular fraction depends on the availability of glucose in the media. When wild-type cells were grown in media containing high glucose, FBPase was not expressed and was not detected in the extracellular fraction. When cells were grown in media containing low glucose for 1 d, FBPase was induced. However, this protein was not detectable in the extracellular fraction. In 2 d-starved cells, low levels of FBPase appeared in the extracellular fraction and FBPase levels in the extracellular fraction increased further in cells that were starved for 3 d.
The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is on the cell surface of Saccharomyces cerevisiae grown in medium containing high glucose.Citation67 As such, substantial amounts of GAPDH were in the extracellular fraction in cells grown in high glucose media. However, levels of extracellular GAPDH decreased in cells grown in low glucose media for 1 and 2 d. Amounts of extracellular GAPDH increased following growth in low glucose medium for 3 d. Thus, the distribution of FBPase and GAPDH in the extracellular fraction changes depending on the availability of glucose in the media.
Vps34p is Critical for the Internalization of Extracellular FBPase in Response to Glucose Addition
Phospholipids and sterols play critical roles in many protein trafficking pathways. The VPS34 gene encodes a class III phosphatidylinositol (PtdIns) 3-kinase (PI3K) which phosphorylates phosphatidylinositol at the 3′ hydroxyl position to produce PtdIns 3-phosphate (PI3P).Citation68-Citation70 The yeast Vps34p is involved in multiple protein trafficking events including endocytosis, sorting of vacuolar proteins, vacuole segregation, multi-vesicular body formation, the cytoplasm to the vacuole pathway and starvation induced macroautophagy.Citation68-Citation70
The VPS34 gene has critical roles in the Vid pathway.Citation64 Vps34p associates with actin patches constitutively. The association requires the ARC18 and SLA1 genes. In cells lacking the VPS34 gene, FBPase and Vid24p constitutively co-localized with actin patches. Hence, the association of Vid vesicles with actin patches persists in the absence of the VPS34 gene.
VPS34 has an important role in the reduction of extracellular FBPase in response to glucose re-feeding. In 3 d-starved Δvps34 mutant, substantial amounts of FBPase were in the periplasm as shown by immuno-TEM. However, following the addition of glucose, most of the FBPase remained in the periplasm. These results were further confirmed using the extraction procedure. In 3 d-starved Δvps34 mutant, a high percentage of FBPase was in the extracellular fraction. Following a transfer of the Δvps34 mutant to media containing high glucose, significant amounts of FBPase remained in the extracellular fraction.Citation64 Taken together, these results indicate that VPS34 is involved in the decline of extracellular FBPase in response to glucose. VPS34, however, is not required for the appearance of FBPase in the periplasm during starvation.
The N736 Residue and the C-terminal 11 Amino Acids of Vps34p are Critical for the Reduction of Extracellular FBPase Following Glucose Addition
The N736 residue of Vps34p is critical for PI3K activity and plays important roles in many vacuole pathways including the Vid pathway.Citation68,Citation71-Citation73 FBPase degradation was retarded in cells harboring the N736K mutation.Citation64 In addition, the N736K mutant protein did not co-localize with actin patches. Moreover, the N736 mutant delayed the clearance of extracellular FBPase in response to glucose re-feeding.Citation64
The C-terminal 11 amino acids of Vps34p (amino acids 864–875) is implicated in the association of Vps34p on the membrane.Citation74,Citation75 FBPase degradation was inhibited in cells in which the C-terminal 11 amino acids were deleted.Citation64 Moreover, the ΔC11 mutant protein and actin patches did not co-localize. In addition, the absence of the C-terminal 11 amino acids retarded the clearance of extracellular FBPase in response to glucose. These results indicate that the N736 residue and the C-terminal 11 amino acids are critical for Vps34p association with actin patches and the reduction of extracellular FBPase following glucose re-feeding.
The Vid Pathway Model
Based on the current knowledge about the Vid pathway, the following model has been proposed (). When cells are grown in low glucose for 3 d, FBPase is secreted into the periplasm. The secretion of FBPase into the extracellular fraction/periplasm increases as cells are starved longer. Interestingly, levels of the glycolytic enzyme GAPDH in the extracellular fraction also depend on the growth conditions. Because FBPase and GAPDH do not contain the signal sequences for the ER-Golgi pathway, these proteins are secreted via the non-classical pathway.
Figure 1. A model for the Vid pathway. When wild-type cells are starved of glucose for a prolonged period of time, significant amounts of FBPase are secreted into the periplasm. Following glucose re-feeding, FBPase is internalized into Vid/endosomes. The internalization of FBPase requires the SLA1, ARC18 and VPS34 genes. Under the same conditions, most of the Vid24p, Sec28p, Vid30p and Vps34p are in the intracellular fraction. Vid24p, Sec28p and Vid30p associate with actin patches initially and dissociate later, whereas Vps34p associates with actin patches constitutively. Following internalization, FBPase is targeted to the vacuole and then degraded in the lumen.
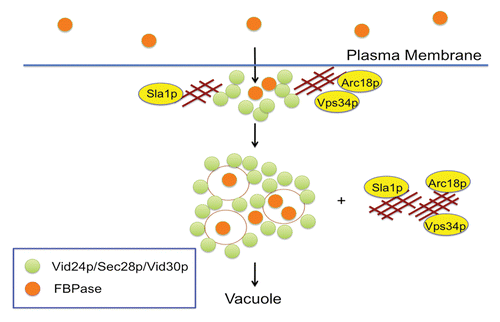
Following a transfer of prolonged-starved cells to high glucose for 15 min, secreted FBPase is internalized rapidly into Vid/endosomes. FBPase internalization enables cells to retrieve most of the extracellular FBPase into the cytoplasm, allowing this protein to be targeted to the vacuole for degradation. FBPase internalization is dependent on the VPS34 gene encoding PI3K.Citation64 Vps34p association with actin patches is linked to the decline of extracellular FBPase. When the C-terminal 11 amino acids were deleted or when the N736 residue was mutated, Vps34p association with actin patches was impaired and the clearance of extracellular FBPase was retarded. Interestingly, most of the extracellular FBPase was cleared in the first 30 min of glucose addition, suggesting that this is a rapid process. FBPase is unlikely to be internalized via the receptor-mediated endocytic pathway due to its lack of a signal sequence. Under the same conditions, molecules involved in the Vid pathway such as Vid24p, Sec28p, Vid30p and Vps34p are retained in the intracellular fraction. In the future, it will be important to elucidate the mechanisms responsible for the secretion and internalization of FBPase via the non-classical pathways.
Prospectives
Why is FBPase secreted in glucose-starved cells and internalized in glucose re-fed cells? One possibility is that FBPase is secreted when the need for this enzyme inside the cells is decreased. FBPase may be in greater demand in the intracellular fraction for 1 d-starved cells. However, the need for FBPase in the intracellular fraction may decrease in 3 d-starved cells. As such, more FBPase is secreted from cells that are starved for 3 d. It has been reported that extracellular GAPDH is enzymatically active.Citation76,Citation77 If metabolic enzymes are secreted as active enzymes, they can perform the same biological functions readily after they are internalized. Secreted proteins may also have additional roles unrelated to their well-known functions inside the cells. For example, GAPDH and enolase participate in host-pathogen interaction during infection by C. albicans.Citation66,Citation67
In yeast, FBPase is associated with Vid vesicles and clusters of Vid vesicles. Vid vesicles are 30–50 nm in diameter and have densities of about 1.2 g/ml. Small vesicles called exosomes are secreted from a variety of mammalian cells.Citation78-Citation80 Exosomes carry a common set of proteins found in most cell types as well as proteins that are unique to the cells that secreted them. Purified exosomes from mammalian cells are 40–100 nm in diameter and have densities of about 1.1–1.2 g/ml.Citation78-Citation80 Moreover, exosomes isolated from mouse MC/9 cells entered human HMC-1 cells and changed protein profiles of the HMC-1 cells,Citation81 suggesting that exosomes can enter recipient cells from a different species.
There are important questions that remain to be addressed. For example, is FBPase secreted in vesicles? If so, is it internalized in vesicles? Are Vid vesicles similar to exosomes? Can Vid vesicles enter other cells? If so, do they fuse directly with the plasma membrane or enter through protein- or vesicle-conducting channels? Are Vid/endosomes similar to the multi-vesicular bodies implicated in endocytosis in mammalian cells? Why are proteins secreted in vesicles? Since both membrane proteins and luminal proteins can be packaged in vesicles, the number of proteins secreted into the extracellular space may increase substantially. Furthermore, vesicles may protect proteins from sudden changes in the environments. For instance, when cells are challenged with toxic materials, surface proteins may be damaged, but luminal proteins may be spared. Given that exosomes contain mRNA and microRNA, they may be involved in the transfer of genetic materials to recipient cells. As such, they have the potential for gene therapy. Therefore, secreted proteins may have multiple roles, to protect cells from toxic materials, to adjust metabolic needs in response to changing environments, to participate in cell-to-cell communication and to transfer genetic materials to recipient cells.
Bacteria, viruses, fungi and parasites secrete a large number of signal-less proteins during infection. Cancer cells also secrete many signal-less proteins during growth and invasion. Defects in endocytosis have also been linked to various neurological disorders.Citation82 For instance, Vps34p is involved in endocytosis in mammalian cellsCitation83 and the deletion of VPS34 in sensory neurons causes neurodegeneration by disrupting the endosomal pathway.Citation82 Thus, understanding the molecular mechanisms responsible for the unconventional secretory and internalizing pathways should have far-reaching impacts on many biological processes such as metabolic regulation, protein trafficking, pathogen infection and cancer growth and invasion.
| Abbreviations: | ||
| FBPase | = | fructose-1,6-bisphosphatase |
| Icl1p | = | isocitrate lyase |
| Pck1p | = | phosphoenolpyruvate carboxykinase |
| MDH2 | = | malate dehydrogenase |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr 2007; 27:19 - 40; http://dx.doi.org/10.1146/annurev.nutr.27.061406.093749; PMID: 17311494
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10:458 - 67; http://dx.doi.org/10.1038/nrm2708; PMID: 19491929
- Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci 2011; 124:161 - 70; http://dx.doi.org/10.1242/jcs.064576; PMID: 21187343
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011; 469:323 - 35; http://dx.doi.org/10.1038/nature09782; PMID: 21248839
- Mijaljica D, Prescott M, Klionsky DJ, Devenish RJ. Autophagy and vacuole homeostasis: a case for self-degradation?. Autophagy 2007; 3:417 - 21; PMID: 17534141
- Platini F, Pérez-Tomás R, Ambrosio S, Tessitore L. Understanding autophagy in cell death control. Curr Pharm Des 2010; 16:101 - 13; http://dx.doi.org/10.2174/138161210789941810; PMID: 20214621
- Wang RC, Levine B. Autophagy in cellular growth control. FEBS Lett 2010; 584:1417 - 26; http://dx.doi.org/10.1016/j.febslet.2010.01.009; PMID: 20096689
- Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 2008; 15:344 - 57; http://dx.doi.org/10.1016/j.devcel.2008.08.012; PMID: 18804433
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 2008; 132:27 - 42; http://dx.doi.org/10.1016/j.cell.2007.12.018; PMID: 18191218
- Monastyrska I, Rieter E, Klionsky DJ, Reggiori F. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc 2009; 84:431 - 48; http://dx.doi.org/10.1111/j.1469-185X.2009.00082.x; PMID: 19659885
- Yang DS, Lee JH, Nixon RA. Monitoring autophagy in Alzheimer’s disease and related neurodegenerative diseases. Methods Enzymol 2009; 453:111 - 44; http://dx.doi.org/10.1016/S0076-6879(08)04006-8; PMID: 19216904
- Cecconi F, Piacentini M, Fimia GM. The involvement of cell death and survival in neural tube defects: a distinct role for apoptosis and autophagy?. Cell Death Differ 2008; 15:1170 - 7; http://dx.doi.org/10.1038/cdd.2008.64; PMID: 18451869
- García-Arencibia M, Hochfeld WE, Toh PP, Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol 2010; 21:691 - 8; http://dx.doi.org/10.1016/j.semcdb.2010.02.008; PMID: 20188203
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov 2007; 6:304 - 12; http://dx.doi.org/10.1038/nrd2272; PMID: 17396135
- Menzies FM, Huebener J, Renna M, Bonin M, Riess O, Rubinsztein DC. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain 2010; 133:93 - 104; http://dx.doi.org/10.1093/brain/awp292; PMID: 20007218
- Minard KI, McAlister-Henn L. Glucose-induced degradation of the MDH2 isozyme of malate dehydrogenase in yeast. J Biol Chem 1992; 267:17458 - 64; PMID: 1324938
- Carlson M. Glucose repression in yeast. Curr Opin Microbiol 1999; 2:202 - 7; http://dx.doi.org/10.1016/S1369-5274(99)80035-6; PMID: 10322167
- Holzer H. Proteolytic catabolite inactivation in Saccharomyces cerevisiae. Revis Biol Celular 1989; 21:305 - 19; PMID: 2561496
- Toyoda Y, Fujii H, Miwa I, Okuda J, Sy J. Anomeric specificity of glucose effect on cAMP, fructose 1,6-bisphosphatase, and trehalase in yeast. Biochem Biophys Res Commun 1987; 143:212 - 7; http://dx.doi.org/10.1016/0006-291X(87)90652-8; PMID: 3030316
- Chiang HL, Schekman R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature 1991; 350:313 - 8; http://dx.doi.org/10.1038/350313a0; PMID: 1848921
- Chiang HL, Schekman R, Hamamoto S. Selective uptake of cytosolic, peroxisomal, and plasma membrane proteins into the yeast lysosome for degradation. J Biol Chem 1996; 271:9934 - 41; http://dx.doi.org/10.1074/jbc.271.17.9934; PMID: 8626630
- Gancedo C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J Bacteriol 1971; 107:401 - 5; PMID: 4329729
- Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev 1998; 62:334 - 61; PMID: 9618445
- Gamo FJ, Navas MA, Blazquez MA, Gancedo C, Gancedo JM. Catabolite inactivation of heterologous fructose-1,6-bisphosphatases and fructose-1,6-bisphosphatase-beta-galactosidase fusion proteins in Saccharomyces cerevisiae. Eur J Biochem 1994; 222:879 - 84; http://dx.doi.org/10.1111/j.1432-1033.1994.tb18935.x; PMID: 8026498
- Tzagoloff A. Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J Biol Chem 1969; 244:5027 - 33; PMID: 4241924
- Lee FJ, Lin LW, Smith JA. A glucose-repressible gene encodes acetyl-CoA hydrolase from Saccharomyces cerevisiae. J Biol Chem 1990; 265:7413 - 8; PMID: 1970569
- Horak J, Regelmann J, Wolf DH. Two distinct proteolytic systems responsible for glucose-induced degradation of fructose-1,6-bisphosphatase and the Gal2p transporter in the yeast Saccharomyces cerevisiae share the same protein components of the glucose signaling pathway. J Biol Chem 2002; 277:8248 - 54; http://dx.doi.org/10.1074/jbc.M107255200; PMID: 11773046
- Horak J, Wolf DH. The ubiquitin ligase SCF(Grr1) is required for Gal2p degradation in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun 2005; 335:1185 - 90; http://dx.doi.org/10.1016/j.bbrc.2005.08.008; PMID: 16112084
- Riballo E, Herweijer M, Wolf DH, Lagunas R. Catabolite inactivation of the yeast maltose transporter occurs in the vacuole after internalization by endocytosis. J Bacteriol 1995; 177:5622 - 7; PMID: 7559351
- Gadura N, Michels CA. Sequences in the N-terminal cytoplasmic domain of Saccharomyces cerevisiae maltose permease are required for vacuolar degradation but not glucose-induced internalization. Curr Genet 2006; 50:101 - 14; http://dx.doi.org/10.1007/s00294-006-0080-3; PMID: 16741702
- Hämmerle M, Bauer J, Rose M, Szallies A, Thumm M, Düsterhus S, et al. Proteins of newly isolated mutants and the amino-terminal proline are essential for ubiquitin-proteasome-catalyzed catabolite degradation of fructose-1,6-bisphosphatase of Saccharomyces cerevisiae. J Biol Chem 1998; 273:25000 - 5; http://dx.doi.org/10.1074/jbc.273.39.25000; PMID: 9737955
- Hung GC, Brown CR, Wolfe AB, Liu J, Chiang HL. Degradation of the gluconeogenic enzymes fructose-1,6-bisphosphatase and malate dehydrogenase is mediated by distinct proteolytic pathways and signaling events. J Biol Chem 2004; 279:49138 - 50; http://dx.doi.org/10.1074/jbc.M404544200; PMID: 15358789
- Shieh HL, Chen Y, Brown CR, Chiang HL. Biochemical analysis of fructose-1,6-bisphosphatase import into vacuole import and degradation vesicles reveals a role for UBC1 in vesicle biogenesis. J Biol Chem 2001; 276:10398 - 406; http://dx.doi.org/10.1074/jbc.M001767200; PMID: 11134048
- Shieh HL, Chiang HL. In vitro reconstitution of glucose-induced targeting of fructose-1, 6-bisphosphatase into the vacuole in semi-intact yeast cells. J Biol Chem 1998; 273:3381 - 7; http://dx.doi.org/10.1074/jbc.273.6.3381; PMID: 9452458
- Jiang Y, Davis C, Broach JR. Efficient transition to growth on fermentable carbon sources in Saccharomyces cerevisiae requires signaling through the Ras pathway. EMBO J 1998; 17:6942 - 51; http://dx.doi.org/10.1093/emboj/17.23.6942; PMID: 9843500
- Regelmann J, Schüle T, Josupeit FS, Horak J, Rose M, Entian KD, et al. Catabolite degradation of fructose-1,6-bisphosphatase in the yeast Saccharomyces cerevisiae: a genome-wide screen identifies eight novel GID genes and indicates the existence of two degradation pathways. Mol Biol Cell 2003; 14:1652 - 63; http://dx.doi.org/10.1091/mbc.E02-08-0456; PMID: 12686616
- Brown CR, Chiang HL. A selective autophagy pathway that degrades gluconeogenic enzymes during catabolite inactivation. Commun Integr Biol 2009; 2:177 - 83; PMID: 19513275
- Brown CR, Cui DY, Hung GG, Chiang HL. Cyclophilin A mediates Vid22p function in the import of fructose-1,6-bisphosphatase into Vid vesicles. J Biol Chem 2001; 276:48017 - 26; PMID: 11641409
- Brown CR, Dunton D, Chiang HL. The vacuole import and degradation pathway utilizes early steps of endocytosis and actin polymerization to deliver cargo proteins to the vacuole for degradation. J Biol Chem 2010; 285:1516 - 28; http://dx.doi.org/10.1074/jbc.M109.028241; PMID: 19892709
- Brown CR, Hung GC, Dunton D, Chiang HL. The TOR complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway. J Biol Chem 2010; 285:23359 - 70; http://dx.doi.org/10.1074/jbc.M109.075143; PMID: 20457600
- Brown CR, Liu J, Hung GC, Carter D, Cui D, Chiang HL. The Vid vesicle to vacuole trafficking event requires components of the SNARE membrane fusion machinery. J Biol Chem 2003; 278:25688 - 99; http://dx.doi.org/10.1074/jbc.M210549200; PMID: 12730205
- Brown CR, McCann JA, Chiang HL. The heat shock protein Ssa2p is required for import of fructose-1, 6-bisphosphatase into Vid vesicles. J Cell Biol 2000; 150:65 - 76; http://dx.doi.org/10.1083/jcb.150.1.65; PMID: 10893257
- Brown CR, McCann JA, Hung GG, Elco CP, Chiang HL. Vid22p, a novel plasma membrane protein, is required for the fructose-1,6-bisphosphatase degradation pathway. J Cell Sci 2002; 115:655 - 66; PMID: 11861771
- Brown CR, Wolfe AB, Cui D, Chiang HL. The vacuolar import and degradation pathway merges with the endocytic pathway to deliver fructose-1,6-bisphosphatase to the vacuole for degradation. J Biol Chem 2008; 283:26116 - 27; http://dx.doi.org/10.1074/jbc.M709922200; PMID: 18660504
- Hoffman M, Chiang HL. Isolation of degradation-deficient mutants defective in the targeting of fructose-1,6-bisphosphatase into the vacuole for degradation in Saccharomyces cerevisiae. Genetics 1996; 143:1555 - 66; PMID: 8844145
- Huang PH, Chiang HL. Identification of novel vesicles in the cytosol to vacuole protein degradation pathway. J Cell Biol 1997; 136:803 - 10; http://dx.doi.org/10.1083/jcb.136.4.803; PMID: 9049246
- Chiang MC, Chiang HL. Vid24p, a novel protein localized to the fructose-1, 6-bisphosphatase-containing vesicles, regulates targeting of fructose-1,6-bisphosphatase from the vesicles to the vacuole for degradation. J Cell Biol 1998; 140:1347 - 56; http://dx.doi.org/10.1083/jcb.140.6.1347; PMID: 9508768
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol 1996; 133:29 - 41; http://dx.doi.org/10.1083/jcb.133.1.29; PMID: 8601610
- Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol 1997; 139:1183 - 95; http://dx.doi.org/10.1083/jcb.139.5.1183; PMID: 9382865
- Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier JL, et al. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 1999; 97:63 - 73; http://dx.doi.org/10.1016/S0092-8674(00)80715-1; PMID: 10199403
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell 1995; 83:703 - 13; http://dx.doi.org/10.1016/0092-8674(95)90183-3; PMID: 8521487
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol 1997; 139:1747 - 59; http://dx.doi.org/10.1083/jcb.139.7.1747; PMID: 9412469
- Alibhoy AA, Giardina BJ, Dunton DD, Chiang HL. Vid30 is required for the association of Vid vesicles and actin patches in the vacuole import and degradation pathway. Autophagy 2012; 8:29 - 46; http://dx.doi.org/10.4161/auto.8.1.18104; PMID: 22082961
- Cui DY, Brown CR, Chiang HL. The type 1 phosphatase Reg1p-Glc7p is required for the glucose-induced degradation of fructose-1,6-bisphosphatase in the vacuole. J Biol Chem 2003; 279:9713 - 24; http://dx.doi.org/10.1074/jbc.M310793200; PMID: 14684743
- Liu J, Brown CR, Chiang HL. Degradation of the gluconeogenic enzyme fructose-1, 6-bisphosphatase is dependent on the vacuolar ATPase. Autophagy 2005; 1:146 - 157; http://dx.doi.org/10.4161/auto.1.3.2036; PMID: 16874049
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 1995; 128:779 - 92; http://dx.doi.org/10.1083/jcb.128.5.779; PMID: 7533169
- Galletta BJ, Cooper JA. Actin and endocytosis: mechanisms and phylogeny. Curr Opin Cell Biol 2009; 21:20 - 7; http://dx.doi.org/10.1016/j.ceb.2009.01.006; PMID: 19186047
- Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 2003; 115:475 - 87; http://dx.doi.org/10.1016/S0092-8674(03)00883-3; PMID: 14622601
- Sekiya-Kawasaki M, Groen AC, Cope MJ, Kaksonen M, Watson HA, Zhang C, et al. Dynamic phosphoregulation of the cortical actin cytoskeleton and endocytic machinery revealed by real-time chemical genetic analysis. J Cell Biol 2003; 162:765 - 72; http://dx.doi.org/10.1083/jcb.200305077; PMID: 12952930
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol 2003; 19:287 - 332; http://dx.doi.org/10.1146/annurev.cellbio.19.111401.093127; PMID: 14570572
- Mizuguchi M, Takashima S, Kakita A, Yamada M, Ikeda K. Lissencephaly gene product. Localization in the central nervous system and loss of immunoreactivity in Miller-Dieker syndrome. Am J Pathol 1995; 147:1142 - 51; PMID: 7573359
- Dobyns WB, Stratton RF, Greenberg F. Syndromes with lissencephaly. I: Miller-Dieker and Norman-Roberts syndromes and isolated lissencephaly. Am J Med Genet 1984; 18:509 - 26; http://dx.doi.org/10.1002/ajmg.1320180320; PMID: 6476009
- Kobayashi N, Yang J, Ueda A, Suzuki T, Tomaru K, Takeno M, et al. RanBPM, Muskelin, p48EMLP, p44CTLH, and the armadillo-repeat proteins ARMC8alpha and ARMC8beta are components of the CTLH complex. Gene 2007; 396:236 - 47; http://dx.doi.org/10.1016/j.gene.2007.02.032; PMID: 17467196
- Alibhoy AA, Giardina BJ, Dunton DD, Chiang HL. Vps34p is required for the decline of extracellular fructose-1,6-bisphosphatase in the vacuole import and degradation pathway. J Biol Chem 2012; 287:33080 - 93; http://dx.doi.org/10.1074/jbc.M112.360412; PMID: 22833678
- Cleves AE, Cooper DN, Barondes SH, Kelly RB. A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol 1996; 133:1017 - 26; http://dx.doi.org/10.1083/jcb.133.5.1017; PMID: 8655575
- Chaffin WL, López-Ribot JL, Casanova M, Gozalbo D, Martínez JP. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev 1998; 62:130 - 80; PMID: 9529890
- Gozalbo D, Gil-Navarro I, Azorín I, Renau-Piqueras J, Martínez JP, Gil ML. The cell wall-associated glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is also a fibronectin and laminin binding protein. Infect Immun 1998; 66:2052 - 9; PMID: 9573088
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 1993; 260:88 - 91; http://dx.doi.org/10.1126/science.8385367; PMID: 8385367
- Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell 2006; 126:191 - 203; http://dx.doi.org/10.1016/j.cell.2006.04.045; PMID: 16839886
- Herman PK, Emr SD. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol 1990; 10:6742 - 54; PMID: 2247081
- Herman PK, Emr SD. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol 1990; 10:6742 - 54; PMID: 2247081
- Kihara ANT, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae.. J Cell Biol 2001; 152:519 - 30; http://dx.doi.org/10.1083/jcb.152.3.519; PMID: 11157979
- Burda P, Padilla SM, Sarkar S, Emr SD. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J Cell Sci 2002; 115:3889 - 900; http://dx.doi.org/10.1242/jcs.00090; PMID: 12244127
- Miller STB, Tavshanjian B, Oleksy A, Perisic O, Houseman BT, Shokat KM, et al. Shaping development of autophagy inhibitors with the structure of the lipid kinase Vps34. Science 2010; 327:1638 - 42; http://dx.doi.org/10.1126/science.1184429; PMID: 20339072
- Budovskaya YVHH, Hama H, DeWald DB, Herman PK. The C terminus of the Vps34p phosphoinositide 3-kinase is necessary and sufficient for the interaction with the Vps15p protein kinase. J Biol Chem 2001; 277:287 - 94; http://dx.doi.org/10.1074/jbc.M109263200; PMID: 11689570
- Delgado ML, O’Connor JE, Azorín I, Renau-Piqueras J, Gil ML, Gozalbo D. The glyceraldehyde-3-phosphate dehydrogenase polypeptides encoded by the Saccharomyces cerevisiae TDH1, TDH2 and TDH3 genes are also cell wall proteins. Microbiology 2001; 147:411 - 7; PMID: 11158358
- Delgado ML, Gil ML, Gozalbo D. Starvation and temperature upshift cause an increase in the enzymatically active cell wall-associated glyceraldehyde-3-phosphate dehydrogenase protein in yeast. FEMS Yeast Res 2003; 4:297 - 303; http://dx.doi.org/10.1016/S1567-1356(03)00159-4; PMID: 14654434
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 2010; 73:1907 - 20; http://dx.doi.org/10.1016/j.jprot.2010.06.006; PMID: 20601276
- Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009; 9:4997 - 5000; http://dx.doi.org/10.1002/pmic.200900351; PMID: 19810033
- Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 2009; 6:267 - 83; http://dx.doi.org/10.1586/epr.09.17; PMID: 19489699
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654 - 9; http://dx.doi.org/10.1038/ncb1596; PMID: 17486113
- Zhou X, Wang L, Hasegawa H, Amin P, Han BX, Kaneko S, et al. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci USA 2010; 107:9424 - 9; http://dx.doi.org/10.1073/pnas.0914725107; PMID: 20439739
- Johnson EE, Overmeyer JH, Gunning WT, Maltese WA. Gene silencing reveals a specific function of hVps34 phosphatidylinositol 3-kinase in late versus early endosomes. J Cell Sci 2006; 119:1219 - 32; http://dx.doi.org/10.1242/jcs.02833; PMID: 16522686