Abstract
Piriformospora indica association has been reported to increase biotic as well as abiotic stress tolerance of its host plants. We analyzed the beneficial effect of P. indica association on rice seedlings during high salt stress conditions (200 and 300 mM NaCl). The growth parameters of rice seedlings such as root and shoot lengths or fresh and dry weights were found to be enhanced in P. indica-inoculated rice seedlings as compared with non-inoculated control seedlings, irrespective of whether they are exposed to salt stress or not. However, salt-stressed seedlings performed much better in the presence of the fungus compared with non-inoculated control seedlings. The photosynthetic pigment content [chlorophyll (Chl) a, Chl b, and carotenoids] was significantly higher in P. indica-inoculated rice seedlings under high salt stress conditions as compared with salt-treated non-inoculated rice seedlings, in which these pigments were found to be decreased. Proline accumulation was also observed during P. indica colonization, which may help the inoculated plants to become salt tolerant. Taken together, P. indica rescues growth diminution of rice seedlings under salt stress.
Introduction
P. indica, a root endophytic filamentous fungus, resides in plant root cortical cells.Citation1P. indica has been classified in the Sebacinales order. The most intriguing character of this beneficial fungus is that it has a broad host spectrum range that includes monocots, eudicots, and dicots.Citation1-Citation10P. indica has been shown to improve the plant growth, nutrient uptake, and helped plants during abiotic and biotic stress tolerance. Because of these virtues, P. indica has proven to be a plant biofertilizer, probiotic, and biohardening tool.Citation11-Citation13P. indica-mediated salt tolerance mechanism was found to be linked strongly with increase in antioxidants under salt stress in barley which attenuates the NaCl-induced lipid peroxidation, metabolic heat efflux, and fatty acid desaturation in barley leaves.Citation10 It has been reported that P. indica significantly elevated the amount of ascorbic acid and increased the activities of antioxidant enzymes in barley roots during salt stress conditions.Citation14 In response to high salt stress, plants have developed a number of mechanisms to counteract high salt stress, such as mineral ion homeostasis and accumulation of solutes such as proline.Citation15 In the present study we analyzed the growth parameters, photosynthetic pigment, and proline contents of P. indica-inoculated and non-inoculated rice seedlings under high salt stress.
Results
P. indica colonization
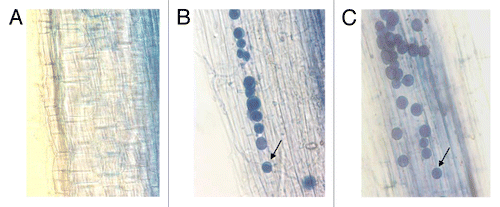
P. indica colonization of rice roots was checked after 15 and 20 d of inoculation (dpi). We have observed colonization at 15 and 20 dpi; however, no colonization was observed in plants that were inoculated using autoclaved P. indica. In plants that were inoculated with the living fungal chlamydospores, we have observed 60 and 70% colonization of the entire root at 15 and 20 dpi, respectively ().
Figure 1.P. indica colonization of rice roots. (A) Negative control inoculated with autoclaved P. indica shows no chlamydspores in the root cortex region. (B) Rice root segment showing colonization at 15 dpi. (C) Root colonization at 20 dpi. Fifteen days after P. indica inoculation, 60% colonization was detected. Control rice plants were mock treated with autoclaved P. indica and contained no spores. Arrow indicates a single chlamydospore of P. indica.
P. indica association improves growth of rice plants
We have found that roots of P. indica-inoculated rice plants were hard, thick, brownish, and higher in number as compared with the non-inoculated plants (). In general, we have observed that P. indica colonization enhanced root and shoot lengths as well as the fresh and dry weights of the host plants (). While the difference between the shoot lengths for non-inoculated and inoculated plants was found to be non significantly different, when the plants were treated with 200 mM NaCl (at day 5 and 15) or 300 mM NaCl (at day 15), the difference in the root lengths was significantly different (p < 0.05) at all times points, except at day 15 when 300 mM NaCl treatment was given. At both 200 and 300 mM NaCl treatment, we have observed that P. indica significantly (p < 0.05) increased the dry weight of the plants at all time points as compared with the non-inoculated plants ().
Figure 2.P. indica colonization. (A) Non-inoculated rice roots were thinner when compared with P. indica-inoculated rice roots. Strong, hard, and brownish roots were observed in P. indica-inoculated rice plants. (B) Root number in 25-d-old control plants and those inoculated by P. indica (15 dpi). Root numbers are higher in case of P. indica-inoculated rice. Each column represents the means of 3 measurements ± Standard Error.
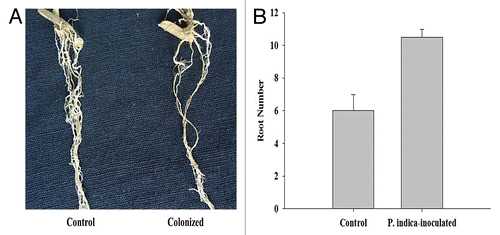
Table 1. Growth parameters of non-inoculated (NI) and P. indica-inoculated (PI) rice seedlings
P. indica-inoculated plants combat with high salt stress in better way
Under high salt conditions P. indica-inoculated rice grows better and remains healthier as compared with the non-inoculated control (). The growth parameters of inoculated rice plants were significantly improved under high salt stress compared with high salt stressed non-inoculated plants. Growth of non-inoculated plants was ceased after a certain period of time and the seedlings were found to become brownish in color.
Figure 3. Rice plants after 10 d salt stress. For representation purposes, one pot per treatment is shown. (A) For 200 mM and (B) for 300 mM salt stress. (a) Non-inoculated rice without salt treatment. (b) P. indica-inoculated rice without salt treatment. (c) Non-inoculated rice treated with 200 (300) mM salt. (d) P. indica-inoculated rice treated with 200 (300) mM salt.

Progression of P. indica colonization affects photosynthetic pigment content of rice plant
A major response of salinity stress in plants is the degradation of photosynthetic pigments, which is caused by chlorosis, reduced photosynthesis, and oxidative damage. As a result, plants become brownish, have stunted growth and reduced weight. Chl a, Chl b, and carotenoid content was measured in inoculated and non-inoculated plants at high salinity stress. A strong effect of the high salt stress was seen at 5 dpi. The photosynthetic pigments decreased much stronger in non-inoculated plants as compared with the P. indica-inoculated plants. P. indica colonization results in higher Chl a levels compared with the non-inoculated controls at all time points (). We have found that inoculated plants have significantly (p < 0.05) higher Chl b at 10 dpi as compared with the non-inoculated plants when 200 mM NaCl treatment was given. Furthermore, P. indica colonization significantly (p < 0.05) resulted in the higher carotenoid content. A 3.15-fold difference was found in the carotenoid content in plants exposed to 300 mM NaCl at 15 dpi, and this difference was found to be significant (p < 0.05) ().
Table 2. Photosynthesis pigments of non-inoculated (NI) and P. indica-inoculated (PI) rice seedlings
Progression of P. indica colonization affects proline content of rice plants
Proline accumulation is an immediate response of plants to combat any type of stress. We have observed that the proline content increased significantly (1.5-fold, p < 0.05) in P. indica-inoculated rice seedlings as compared with the non-inoculated plants when 200 mM NaCl treatment was given (). Interestingly, we also observed an enhanced proline content in P. indica-inoculated rice plants that are not exposed to salt stress as compared with the non-inoculated plants. Without salt stress, an approximately 6-fold difference in proline content was detected between P. indica-inoculated and control plants. This suggests that P. indica-inoculated seedlings become more salt tolerant because of higher proline accumulation ().
Table 3. Total proline content of non-inoculated (NI) and P. indica-inoculated (PI) rice seedlings under 200 mM salt stress (SS)
Discussion
In the present study, we used rice plants to show higher resistibility against salinity stress upon colonization with P. indica. Salinity is one of the major abiotic stress that affects plants growth, development, and productivity. Here, we demonstrate that important physiological parameters that are related to growth, photosynthesis, and stress-induced metabolite production are impaired under salt stress in rice plants.
Growth analysis is a fundamental characteristic to study plant’s response to an environmental stress. Salt stress induces stomatal closure, declines photosynthesis, induces photoinhibition, and disrupts nutrient balance by affecting the availability, transport, and partitioning of nutrients.Citation16 Thus, an immediate effect of salt stress causes lower body mass, stunted growth, and reduced chlorophyll concentration. To investigate the contribution of P. indica in protecting the plant against salinity stress, we inoculated rice plants with the fungus. We measured dry and fresh weights, root, and shoot lengths of inoculated plants grown in normal soil and compare the data with the non-inoculated plants grown under the same condition. We found that inoculated plants show significantly higher growth rates than non-inoculated plants. In our previous study by Kumar et al.,Citation17 we also observed a growth promoting activity of P. indica on plants by providing essential mineral nutrients, mainly phosphate, to plants. Here, we report for the first time a significant effect of P. indica on growth of a salt sensitive variety of rice under high salt stress. We found that upon colonization, rice plants can withstand up to 300 mM NaCl and show significantly higher growth than non-inoculated plants. Root and shoot lengths are also found to be higher as compared with non-inoculated plants. Root lengths are important parameters for salt stress because the roots are in direct contact with soil and absorb water from soil.Citation20
Photosynthesis is important for growth and development of green plants and can be severely affected by environmental stress. In the present study, we focused on Chl and carotenoid levels, since they play a vital role in photosynthesis and photoprotection. Chl and carotenoids can be degraded due to high sodium ion toxicity during salt stress. Several crops have been reported of having reduced Chl a and Chl b concentrations upon salinity stress, e.g., cabbage (Brassica oleracea capitata L.), sunflower (Heliantus annuus L.), wheat (Triticum aestivum L.), and sugarcane.Citation18-Citation24 The extent of reduction of the pigment content depends on the salt tolerance of the plant species. In case of some plant species (wheat, pea, sunflower, etc.), the Ch content is a potential biochemical indicator of salt tolerance, although this is not true for all species and cultivars.Citation23 In a study with sugarcane, Gomathi and RakkiyapanCitation24 found that salt stress at various plant growth stages caused a marked reduction in Chl and carotenoid contents, but salt-tolerant varieties exhibited higher membrane stability and pigment contents. We measured the content of Chl a, Chl b, and carotenoids in 200- and 300-mM salt-treated plants with P. indica inoculation. The amount of these pigments was significantly higher in inoculated plants as compared with the non-inoculated control grown under the same salt conditions. Therefore, P. indica helps rice plants by protecting them against salt stress.
The most detrimental role of salinity stress in plants is high ion toxicity and osmotic misbalance. High level of ion accumulation during salinity stress causes the disruption of osmotic balance, membrane disorganization, reduced enzyme activities, breakdown of protein, and metabolic toxicity inside the plant cell. Osmolytes are considered as the major component to maintain osmotic balance inside the cell and protect plants from potential toxic damage. When exposed to stressful conditions, plants accumulate an array of metabolites, particularly amino acids. Proline, an amino acid, plays a highly beneficial role in plants exposed to various stress conditions. Besides acting as an excellent osmolyte, it can also work as a metal chelator, an antioxidative defense molecule, and a signaling molecule. Stressful environment results in an overproduction of proline in plants, which in turn imparts stress tolerance by maintaining cell turgor or osmotic balance, by stabilizing membranes thereby preventing electrolyte leakage, and by maintaining the reactive oxygen species level within normal ranges, thus preventing oxidative burst in plants.Citation25 In this study we have observed that proline accumulated significantly higher in inoculated rice plants as compared with non-inoculated plants during the high salt treatment. Plants inoculated by P. indica survived better under high salt treatment, which indicates that P. indica is helping the plant in maintaining the osmotic pressure. However, it needs more detailed studies to completely understand the mechanism of how P. indica is helping the plant in maintaining osmotic balance. Most studies concerned with arbuscular mycorrhiza-saline soil interactions do not simulate field conditions.Citation26 Our study supports the idea of using and testing P. indica in field conditions.
Materials and Methods
Plant and fungal culture and growth conditions
Seeds of rice (Pusa basmati-1, IARI) were surface-sterilized for 15 min in ethanol followed by dipping for 20 min in a 4% NaClO solution, and finally washed 6 times with sterile water. Seeds were cold treated and germinated on water-agar plates (0.8% Bacto Agar, Difco) at 25°C in the dark for 3 d. Seedlings were placed in pots (9 cm height by 10 cm diameter) containing sand (2–4 mm diameter). Plants were weekly supplied with half-strength modified Hoagland solution containing the following composition: 5 mM KNO3, 5 mM Ca(NO3)2, 2 mM MgSO4, 10 µM KH2PO4, 10 μM MgCl2, 4 μM ZnSO4, 1 μM CaSO4, 1 μM NaMoO4, 50 μM H3BO3. P. indica was cultured on Aspergillus minimal media for 7 d as described previously.Citation27
P. indica colonization
Three-day-old germinated seedlings were planted in pots without P. indica and allowed to grow for 7 d. Rice seedlings were taken out, and the roots were washed and were inoculated with P. indica with sterile sand mixed with the fungal culture (1% fungus in sand by w/w). Control plants were mock inoculated with autoclaved (dead) P. indica-mixed sand. Plants were given half strength Hoagland’s nutrient solution weekly. P. indica colonization was checked 15 and 20 dpi under the light microscope (Leica type 020–518.500) as described previously.Citation28 In brief, colonization was checked by taking 10 root samples randomly from 3 different inoculated rice plants 15 dpi and 20 dpi, i.e., when the rice seedlings were 25 and 30 d old, respectively. Samples were softened in 10% KOH solution for 15 min, acidified with 1 M HCl for 10 min, and finally stained with 0.02% Trypan blue overnight.Citation28-Citation30 Samples were distained with 50% lacto-phenol for 1–2 h prior to observation under the light microscope. The distribution of intracellular chlamydospores within the cortex region of root was taken as an indication of colonization.Citation8 The percentage colonization of the full length root was calculated for the inoculated plants according to a published method.Citation31
Salt treatment and growth analysis of rice
Salt treatment was given when rice seedlings were 10 d old. In case of P. indica-inoculated plants, salt treatment was given in parallel. Four sets of plants were analyzed in this study to check the role of P. indica during salt treatment: 1) Non-inculated plants without salt treatment, 2) plants inoculated with P. indica and salt treatment, 3) plants inoculated with P. indica without salt treatment, and 4) non-inoculated plants with salt treatment. In case of P. indica-inoculated plants, salt stress was given at day 10, and this day was taken as day 0 of salt stress. Two-hundred- and 300-mM NaCl solutions were prepared in 1 × half strength Hoagland solution. Pots having 6–10 plants were placed in trays with different salt solutions.Citation32 Rice root numbers and length, shoot length, fresh and dry weight of P. indica-inoculated and non-inoculated plants were measured after 5, 10, and 15 d of parallel salt treatments.
Determination of chlorophyll a, b and carotenoids contents
Leaves were harvested, weighed, and ground in 90% ammonical acetone (acetone: water: 0.1 N ammonia, ratio of 90: 9: 1) at 4°C. Pigments contents were measured at 663, 645, and 470 nm for Chl a, Chl b, and carotenoids, respectively, using the supernatant. Total Chl content was measured by spectrophotometer and calculated as nmol/ml.Citation33 Chl a = (14.21 × OD663 – 3.01 × OD645), Chl b = (25.23 × OD645 – 5.16 × OD663), Chl (a+ b) = (9.05 × OD663 + 22.2 × OD645), and carotenoids = {1000 × OD470 – (3.27 × Chl a – 1.04 × Chl b)/5}. The obtained values were divided by leaf fresh weight to obtain values in nmol/ml/mg of leaf fresh weight.
Proline content measurement
Proline accumulation is one of the immediate plant responses to stress, therefore the proline content was measured at day 0, 1, 2, 4, 5, 10, and 15. Plants were 10, 11, 12, 14, 15, 20, and 25 d old at these measuring points when P. indica inoculation or salt treatment or both treatments or none of them were applied. Proline content was determined according to method described previously.Citation34 In brief, 0.5 g of plant material was homogenized in 10 ml of 3% aqueous sulfosalicylic acid and the homogenate was centrifuged (10000 rpm for 10 min). The supernatant was reacted with 2 ml acid ninhydrin and 2 ml of glacial acetic acid in a capped test tube for 1 h at 100°C and the reaction terminated in an ice bath. The reaction mixture was extracted with 4 ml ice-cold toluene and mixed vigorously with a test tube glass stirrer for 15–20 s. The chromophore containing toluene was extracted from the aqueous phase, warmed to room temperature, and the absorbance was read at 520 nm using toluene as blank. Proline concentration was determined from a standard curve and calculated on a fresh weight basis as follows: [(µg proline /ml*ml toluene) /115.5 µg/µmole] /(g sample /5) = µmole proline / g of fresh weight material.Citation34
Statistical analysis
For the analyses, SigmaPlot 11.0 version was used. Paired t-test was used to check the significance difference between non-inoculated and P. indica-inoculated plants as well as when the salt treatment was given in both the conditions.
| Abbreviations: | ||
| Chlorophyll (chl) | = | Piriformospora indica (P. indica) |
Acknowledgments
Johri AK and Dua M are thankful to the JNU for providing DST-PURSE and capacity building grant. Jogawat A, Bakshi M, Dayaman V, MK and Saha S are very thankful to CSIR, UGC, ICMR, and DST, Government of India for granting research fellowship.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Verma S, Varma A, Rexer K, Hassel A, Kost G, Sarbhoy A, Bisen P, Bütehorn B, Franken P. Piriformospora indica, gen. et sp. nov, a new root-colonizing fungus. Mycologia 1998; 90:896 - 903; http://dx.doi.org/10.2307/3761331
- Peskan-Berghöfer T, Shahollari B, Giong PH, Hehl S, Markert C, Blanke V, Kost G, Varma A, Oelmüller R. Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant-microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plant 2004; 122:465 - 77; http://dx.doi.org/10.1111/j.1399-3054.2004.00424.x
- Pham GH, Kumari R, Singh A, Sachdev M, Prasad R, Kaldorf M, Buscot F, Oelmüller R, Peskan T, Weiss M, Hampp R, Varma A. Axenic cultures of Piriformospora indica. In: Varma A, Abbott L, Werner D, Hampp R, eds. Plant Surface Microbiology. Springer-Verlag 2004; 593-616.
- Sahay NS, Varma A. Piriformospora indica: a new biological hardening tool for micropropagated plants. FEMS Microbiol Lett 1999; 181:297 - 302; http://dx.doi.org/10.1111/j.1574-6968.1999.tb08858.x; PMID: 10585552
- Shahollari B, Varma A, Oelmüller R. Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J Plant Physiol 2005; 162:945 - 58; http://dx.doi.org/10.1016/j.jplph.2004.08.012; PMID: 16146321
- Shahollari B, Vadassery J, Varma A, Oelmüller R. A leucine-rich repeat protein is required for growth promotion and enhanced seed production mediated by the endophytic fungus Piriformospora indica in Arabidopsis thaliana.. Plant J 2007; 50:1 - 13; http://dx.doi.org/10.1111/j.1365-313X.2007.03028.x; PMID: 17397506
- Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J Biol Chem 2005; 280:26241 - 7; http://dx.doi.org/10.1074/jbc.M500447200; PMID: 15710607
- Varma A, Savita Verma, Sudha, Sahay N, Butehorn B, Franken P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol 1999; 65:2741 - 4; PMID: 10347070
- Varma A, Singh A, Sudha Sahay N, Sharma J, Roy A, Kumari M, Rana D, Thakran S, Deka D, Bharti K, et al. Piriformospora indica: a cultivable mycorrhiza-like endosymbiotic fungus. In: Hock B, eds. Mycota IX. Germany: Springer Series, Berlin. New York: Heidelberg, 2001, 123-150.
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci U S A 2005; 102:13386 - 91; http://dx.doi.org/10.1073/pnas.0504423102; PMID: 16174735
- Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol 2010; 167:1009 - 17; http://dx.doi.org/10.1016/j.jplph.2010.02.013; PMID: 20471134
- Aschheim K. Research highlights: Plant probiotic. Nat Biotechnol 2005; 23:1241; http://dx.doi.org/10.1038/nbt1005-1241
- Stein E, Molitor A, Kogel KH, Waller F. Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol 2008; 49:1747 - 51; http://dx.doi.org/10.1093/pcp/pcn147; PMID: 18842596
- Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel KH, Schäfer P, Schwarczinger I, et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol 2008; 180:501 - 10; http://dx.doi.org/10.1111/j.1469-8137.2008.02583.x; PMID: 18681935
- Lutts S, Majerus V, Kinet JM. NaCl effects on proline metabolism in rice (Oryza sativa L.) seedling. Physiol Plant 1999; 105:450 - 8; http://dx.doi.org/10.1034/j.1399-3054.1999.105309.x
- Jouyban Z. The effects of salt stress on plant growth. Tech J Engin & App Sci 2012; 2:7 - 10
- Kumar M, Yadav V, Kumar H, Sharma R, Singh A, Tuteja N, Johri AK. Piriformospora indica enhances plant growth by transferring phosphate. Plant Signal Behav 2011; 6:723 - 5; http://dx.doi.org/10.4161/psb.6.5.15106; PMID: 21502815
- Jamil M, Lee KB, Jung KY, Lee DB, Han MS, Rha ES. Salt stress inhibits germination and early seedling growth in cabbage (Brassica oleracea capitata L.). Pak J Biol Sci 2007; 10:910 - 4; http://dx.doi.org/10.3923/pjbs.2007.910.914; PMID: 19069887
- Ashraf M, Sultana R. Combination effect of NaCl salinity and N-form on mineral composition of sunflower plants. Biol Plant 2000; 43:615 - 9; http://dx.doi.org/10.1023/A:1002860202032
- Akram NA, Ashraf M. Improvement in growth, chlorophyll pigments and photosynthetic performance in salt-stressed plants of sunflower (Helianthus annuus L.) by foliar application of 5-aminolevulinic acid. Agrochimica 2011; 55:94 - 104
- Arfan M, Athar HR, Ashraf M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress?. J Plant Physiol 2007; 164:685 - 94; http://dx.doi.org/10.1016/j.jplph.2006.05.010; PMID: 16884826
- Perveen S, Shahbaz M, Ashraf M. Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt stressed and non-stressed wheat plants raised from seed treated with triacontanol. Pak J Bot 2010; 42:3073 - 81
- Ashraf M. Harris PJC. Photosynthesis under stressful environments: An overview. Photosynthetica 2013; 51:163 - 90; http://dx.doi.org/10.1007/s11099-013-0021-6
- Gomathi R, Rakkiyapan P. Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int J. Plant Physiol Biochem 2011; 3:67 - 74
- Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments: a review. Plant Signal Behav 2012; 7:1456 - 66; http://dx.doi.org/10.4161/psb.21949; PMID: 22951402
- Al-Karaki GN, Hammad R, Rusan M. Response of two cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza 2001; 11:43 - 7; http://dx.doi.org/10.1007/s005720100098
- Käfer E. Mitotic crossing over and nondisjunction in translocation heterozygotes of Aspergillus.. Genetics 1976; 82:605 - 27; PMID: 773747
- Kumar M, Yadav V, Tuteja N, Johri AK. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 2009; 155:780 - 90; http://dx.doi.org/10.1099/mic.0.019869-0; PMID: 19246749
- Dickson S, Smith SM. Evaluation of vesicular-arbuscular mycorrhizal colonization by staining. In: Varma A, eds. Mycorrhiza Manual. Berlin, Germany: Springer-Verlag, 1998: 77-84.
- Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and VAM fungi for rapid assessment of infection. Trans Br Mycol Soc 1970; 55:158 - 61; http://dx.doi.org/10.1016/S0007-1536(70)80110-3
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 1990; 15:495 - 501; http://dx.doi.org/10.1111/j.1469-8137.1990.tb00476.x
- Rodriguez RJ, Henson J, Van Volkenburgh E, Hoy M, Wright L, Beckwith F, Kim YO, Redman RS. Stress tolerance in plants via habitat-adapted symbiosis. ISME J 2008; 2:404 - 16; http://dx.doi.org/10.1038/ismej.2007.106; PMID: 18256707
- Mane AV, Karadge BA, Samant JS. Salinity induced changes in photosynthetic pigments and polyphenols of Cymbopogon nardus (L.) Rendle. J Chem Pharm Res 2010; 2:338 - 47
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil 1973; 39:205 - 7; http://dx.doi.org/10.1007/BF00018060