Abstract
PtrMAN6 is a plant mannan endo-hydrolase involved in modulating cell expansion and cell wall thickening in Populus developing xylem. N-glycosylation and dimerization affect the PtrMAN6 enzymatic activity, which is crucial for production of the endogenous galactoglucomannan oligosaccharide signal molecule in plants. There are 5 potential N-glycosylation sites and 6 cysteines in PtrMAN6 sequence. Each of the N-glycosylation or cysteine sites was site-direct mutagenized individually as well as in combination to analyze their effects on the PtrMAN6 N-glycosylation or dimerization status and the enzyme activity. Our results demonstrated that all 5 potential N-glycosylation sites are involved in the N-glycosylation, which is essential for PtrMAN6 enzyme activity. Meanwhile, we found only 3 carboxyl-terminal cysteines are involved in formation of disulfide-linked dimer to regulate PtrMAN6 activity. The 3 carboxyl-terminal cysteines were conserved in the wall-bounded mannan endo-hydrolases, and this structure may play a role in regulating the PtrMAN6 activity through interaction with redox signals such as reactive oxygen species (ROS) and hydrogen sulfide (H2S) for GGMOs signal generation.
Keywords: :
Galactoglucomannan oligosaccharides (GGMOs), a set of oligosaccharides derived from mannan-type hemicellulose, exhibit a wide range of biological activities in the process of plant tissue or cell cultures.Citation1-Citation4 Recently, we found that a plant mannan endo-hydrolase PtrMAN6 is able to hydrolyze mannan-type polysaccharides of cell wall and to produce endogenous GGMOs that serve as signaling molecules to modulate the expression of the transcription factor genes that govern cell wall thickening.Citation5 Moreover, the PtrMAN6 enzymatic activity is regulated strictly by both N-glycosylation and dimerization.Citation5 Here, we present a detailed analysis of the co- and post-translational modification of PtrMAN6 and discuss the possible signaling crosstalk mediated by PtrMAN6.
N-glycosylation is important for PtrMAN6 enzymatic activity
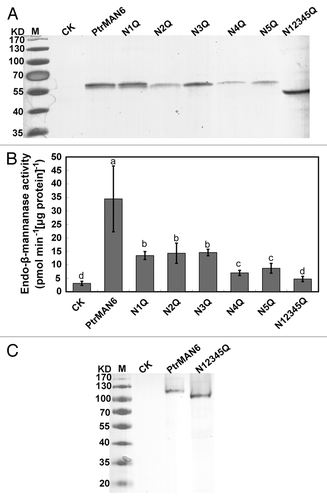
N-glycosylation is the process of covalent attachment of sugars to a nitrogen atom in an amino acid residue (most likely asparagine) in a protein found in eukaryotic organisms. The presence of N-glycans on a protein has substantial effects on its size, structure, and function.Citation6,Citation7 PtrMAN6 is a plasma membrane protein containing 5 potential N-glycosylation sites (Asn-X-Ser/Thr, X can be any amino acid except proline) in its extracellular domain.Citation5 Moreover, deglycosylation with endoglycosidases significantly decreased the PtrMAN6 hydrolase activity, suggesting that N-glycosylation plays an important role in PtrMAN6 enzyme activity.Citation5 To characterize the precise role of the N-glycosylation sites on PtrMAN6 glycosylation and enzyme activity, each potential N-glycosylation site was site-direct mutagenized in individual as well as in combination, where asparagine was changed to glutamine. Then the wild type PtrMAN6 and these mutated genes were placed under CaMV 35S promoter and transiently expressed in Nicotiana benthamiana leaves, and the glycosylation status and the activity of these recombinant PtrMAN6 proteins were assessed. On SDS-PAGE based immunoblot analysis, combinational mutation of the potential 5 sites in PtrMAN6 significantly caused the protein size decrease, while individual mutation of N-glycosylation sites did not show much change (). However, the mutation of Asn-23, Asn-194, Asn-227, Asn-375, or Asn-392 resulted in significantly lower PtrMAN6 enzymatic activity (). Moreover, the combinational mutation of the potential 5 sites completely abolished N-glycosylation of PtrMAN6 ( and see ref. Citation5) and its catalytic activity (). The results demonstrated that all 5 potential N-glycosylation sites are involved in the N-glycosylation, which is essential for PtrMAN6 catalytic activity.
Figure 1. N-glycosylation is essential for PtrMAN6 enzyme activity. (A) Equal volumes of total proteins from tobacco leaves with transient transformation of empty vector (CK), wild type PtrMAN6 (PtrMAN6), or N-glycosylation site mutants were electrophoresed on 10% SDS-PAGE gels under reducing condition and detected with immunoreactions of anti-PtrMAN6 IgG. N1Q, N2Q, N3Q, N4Q, and N5Q indicate changes of Asn-23, Asn-194, Asn-227, Asn-375, and Asn-392 to Gln mutations, respectively. N12345Q indicates the combinational mutation of the 5 sites. (B) Effect of Asn to Gln mutant on PtrMAN6 activity. Proteins were extracted from transformed tobacco leaves and their activity was determined according to the previous study.Citation5 Different letters indicate significant differences at p < 0.05 level by LSD test. Error bars represent the SE of 3 measurements. (C) N-glycosylation deficiency of PtrMAN6 has no effect on its dimerization. Total protein from tobacco leaves was detected with immunoreactions of anti-PtrMAN6 IgG under non-reducing condition.
Three carboxyl-terminal cysteines affect the redox-dependent dimerization and PtrMAN6 activity
In addition to glycosylation, formation of disulfide bonds, both intramolecular and intermolecular, is also an important modification for proper protein folding and secretion.Citation8,Citation9 In our previous study, the native PtrMAN6 forms a disulfide-linked dimer that can be converted to a monomer by reducing conditions. Dimerization of PtrMAN6 is crucial for its catalytic activity.Citation5 However, it is unknown about how PtrMAN6 is dimerized. Here, we reported a characterization of PtrMAN6 dimerization. The results showed that under non-reducing condition, mutants of PtrMAN6 N-glycosylation sites displayed dimer format (), suggesting that N-glycosylation did not affect dimerization of PtrMAN6. There are 6 cysteine sites in the PtrMAN6 that can potentially form disulfide bonds. Each cysteine site in PtrMAN6 was site-direct mutagenized individually and in combination, where cysteine was changed to alanine. These genes were placed under CaMV 35S promoter and transiently expressed in N. benthamiana leaves, then the dimerization status and catalytic activity of the recombinant PtrMAN6 were assessed. Immunoblot analysis revealed that dimerization status of recombinant PtrMAN6 with Cys28Ala (C1A), Cys125Ala (C2A), Cys247Ala (C3A), or combined 3-site mutation (C123A) was the same as that of wild type PtrMAN6, which formed a disulfide-linked dimer under non-reducing condition. In contrast, PtrMAN6 monomer appeared when each 3 carboxyl-terminal cysteines Cys448, Cys452, and Cys456 was mutated to Ala (C4A, C5A, and C6A), and significantly increased when they were mutated in 2-sites combination (C45A, C46A, and C56A), and furthermore, the dimer was completely abolished when all the 3 carboxyl-terminal cysteines were simultaneously mutated (C456A) (). Consistent with the dimerization status, the wide type PtrMAN6 protein displayed higher enzymatic activity. Cys28Ala, Cys125Ala, and Cys247Ala mutations did not affect PtrMAN6’s activity (). In contrast, the catalytic activity of recombinant PtrMAN6-Cys448Ala, Cys452Ala, or Cys456Ala was decreased significantly by 65–90%, and the double mutants of the 3 carboxyl-terminal cysteines resulted in further decreased catalytic activity. The 3 carboxyl-terminal cysteines triple mutant completely lost the enzyme activity (). These results indicated that the 3 carboxyl-terminal cysteines are invoved in formation of disulfide-linked dimer, which is critical for the PtrMAN6 activity.
Figure 2. Carboxyl-terminal 3 cysteines affect PtrMAN6 disulfide-linked dimerization and enzyme activity. (A) Equal volumes of total proteins from tobacco leaves with transient tranformation of empty vector (CK), wild type PtrMAN6 (PtrMAN6), or Cys to Ala mutants were electrophoresed on 10% SDS-PAGE gels under non-reducing condition and detected by immunoreactions of anti-PtrMAN6 IgG. C1A, C2A, C3A, C4A, C5A, and C6A indicate changes of Cys28, Cys125, Cys247, Cys448, Cys452, and Cys456 to Ala mutant, respectively.C45A, C46A, and C56A indicate 2-site combined mutants. C123A and C456A indicate 3-site combined mutants. Dimers and monomers were indicated by arrow. (B) Effect of Cys to Ala mutant on PtrMAN6 activity. Proteins were extracted from tranformed tobacco leaves and their activity was determined according to the previous study.Citation5 Different letters indicate significant differences at p < 0.05 level by LSD test. Error bars represent the SE of 3 measurements. (C) Phylogenetic analysis of endo-β-mannanases from Arabidopsis, Populus, and tomato. Multiple sequence alignments were performed using ClustalW software, and phylogenetic tree was constructed by MEGA5.1 with the neighbor-joining method. Bootstrap values were calculated from 1000 trials. A special group was highlighted. (D) Carboxyl-terminal cysteine repeat motif is conserved in several MAN members. The sequences of PtrMAN6, PtrMAN4, and AtMAN6 were aligned with tomato LeMAN4a. The cysteine repeat motif is boxed and cysteine positions are indicated with asterisks.
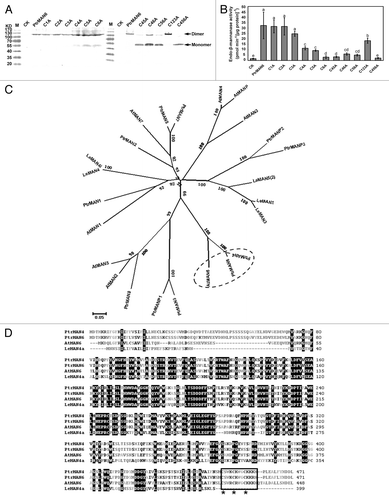
Interestingly, LeMAN in tomato is active as a monomer and its dimerization was not studied.Citation10-Citation12 Alignment analysis of MAN protein sequences from Arabidopsis, Populus, and tomato showed that 2 Populus proteins, PtrMAN6 and PtrMAN4, and 1 Arabidopsis protein, AtMAN6, were clustered to a specific clade (). In this clade, proteins have a conserved carboxyl-terminal cysteine-repeat motif “CSWK/RCKWGCKKK/R” which was absent in all tomato LeMAN proteins (). This motif does not locate in the conserved catalytic domain of MAN proteins (), and is distant from the proposed catalytic cleft in amino acids sequence.Citation11 Because it is a highly hydrophilic group that is supposed to locate on the surface of the proteins, we hypothesize that the cysteine-repeat motif may have served as a cover to block polymer substrates into the catalytic cleft in the monomer status, and the cover is unfolded by its dimerization. Certainly, this hypothesis needs to be tested by further structural analysis for active MAN protein from the specific subgroup. Nevertheless, the results here suggest that the enzyme activity of MANs from this subgroup is regulated by disulfide-linked dimerization via their carboxyl-terminal repeated cysteines.
The disulfide-linked PtrMAN6 dimerization can be regulated by redox agents. Thus, PtrMAN6 dimerization may be in association with local redox status in planta, which can convey spatial information to establish a developmental program. Recently, increasing evidence suggests that several oxidizers such as reactive oxygen species (ROS) and reducers such as hydrogen sulfide (H2S) may be signaling molecules to mediate plant stress response and certain developmental processes including cell wall formation.Citation13-Citation16 In our studies, the GGMOs produced by PtrMAN6 catalysis may act as signaling molecules regulating secondary cell wall thickening during Populus xylem differentiation. Hence, PtrMAN6 may link these redox signals to GGMOs signal controlling secondary cell wall formation through the redox-dependent enzymatic activity regulation. And further characterization of the PtrMAN6 regulation mechanism will provide a full picture of the crosstalk among these signals.
| Abbreviations: | ||
| GGMOs | = | Galactoglucomannan oligosaccharides |
| MAN | = | mannan endo-hydrolase |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Key Basic Research Program of China (2012CB114502), the National Natural Science Foundation of China (31130012), and Shanghai Science and Technology Commission (11XD1405900) to Li L.
References
- Auxtova O, Liskova D, Kakoniova D, Kubackova M, Karacsonyi S, Bilisics L. Effect of Galactoglucomannan-Derived Oligosaccharides on Elongation Growth of Pea and Spruce Stem Segments Stimulated by Auxin. Planta 1995; 196:420 - 4; http://dx.doi.org/10.1007/BF00203638
- Benová-Kákosová A, Digonnet C, Goubet F, Ranocha P, Jauneau A, Pesquet E, Barbier O, Zhang Z, Capek P, Dupree P, et al. Galactoglucomannans increase cell population density and alter the protoxylem/metaxylem tracheary element ratio in xylogenic cultures of Zinnia. Plant Physiol 2006; 142:696 - 709; http://dx.doi.org/10.1104/pp.106.085712; PMID: 16891547
- Kollárová K, Vatehová Z, Slováková L, Lisková D. Interaction of galactoglucomannan oligosaccharides with auxin in mung bean primary root. Plant Physiol Biochem 2010; 48:401 - 6; http://dx.doi.org/10.1016/j.plaphy.2010.03.009; PMID: 20400322
- Richterová-Kučerová D, Kollárová K, Zelko I, Vatehová Z, Lišková D. How do galactoglucomannan oligosaccharides regulate cell growth in epidermal and cortical tissues of mung bean seedlings?. Plant Physiol Biochem 2012; 57:154 - 8; http://dx.doi.org/10.1016/j.plaphy.2012.05.014; PMID: 22705590
- Zhao Y, Song D, Sun J, Li L. Populus endo-beta-mannanase PtrMAN6 plays a role in coordinating cell wall remodeling with suppression of secondary wall thickening through generation of oligosaccharide signals. Plant J 2013; 74:473 - 85; http://dx.doi.org/10.1111/tpj.12137; PMID: 23384057
- Imperiali B, O’Connor SE. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol 1999; 3:643 - 9; http://dx.doi.org/10.1016/S1367-5931(99)00021-6; PMID: 10600722
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 1993; 3:97 - 130; http://dx.doi.org/10.1093/glycob/3.2.97; PMID: 8490246
- Wedemeyer WJ, Welker E, Narayan M, Scheraga HA. Disulfide bonds and protein folding. Biochemistry 2000; 39:7032; http://dx.doi.org/10.1021/bi005111p; PMID: 10841785
- Wittrup KD. Disulfide bond formation and eukaryotic secretory productivity. Curr Opin Biotechnol 1995; 6:203 - 8; http://dx.doi.org/10.1016/0958-1669(95)80033-6; PMID: 7734749
- Bourgault R, Bewley JD. Variation in its C-terminal amino acids determines whether endo-beta-mannanase is active or inactive in ripening tomato fruits of different cultivars. Plant Physiol 2002; 130:1254 - 62; http://dx.doi.org/10.1104/pp.011890; PMID: 12427992
- Bourgault R, Oakley AJ, Bewley JD, Wilce MC. Three-dimensional structure of (1,4)-beta-D-mannan mannanohydrolase from tomato fruit. Protein Sci 2005; 14:1233 - 41; http://dx.doi.org/10.1110/ps.041260905; PMID: 15840830
- Rodríguez-Gacio MdelC, Iglesias-Fernández R, Carbonero P, Matilla AJ. Softening-up mannan-rich cell walls. J Exp Bot 2012; 63:3976 - 88; http://dx.doi.org/10.1093/jxb/ers096; PMID: 22553284
- Choudhury S, Panda P, Sahoo L, Panda SK. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 2013; 8:8; http://dx.doi.org/10.4161/psb.23681; PMID: 23425848
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003; 422:442 - 6; http://dx.doi.org/10.1038/nature01485; PMID: 12660786
- Zhang H, Tan ZQ, Hu LY, Wang SH, Luo JP, Jones RL. Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J Integr Plant Biol 2010; 52:556 - 67; http://dx.doi.org/10.1111/j.1744-7909.2010.00946.x; PMID: 20590986
- Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 2008; 50:1518 - 29; http://dx.doi.org/10.1111/j.1744-7909.2008.00769.x; PMID: 19093970