Abstract
The vein networks of plant leaves are among the most spectacular expressions of biological pattern, and the principles controlling their formation have continually inspired artists and scientists. Control of vein patterning by the polar, cell-to-cell transport of the plant signaling molecule auxin—mediated in Arabidopsis primarily by the plasma-membrane-localized PIN1—has long been known. By contrast, the existence of intracellular auxin transport and its contribution to vein patterning are recent discoveries. The endoplasmic-reticulum-localized PIN5, PIN6, and PIN8 of Arabidopsis define an intracellular auxin-transport pathway whose functions in vein patterning overlap with those of PIN1-mediated intercellular auxin transport. The genetic interaction between the components of the intracellular auxin-transport pathway is far from having been resolved. The study of vein patterning provides experimental access to gain such a resolution—a resolution that in turn holds the promise to improve our understanding of one of the most fascinating examples of biological pattern formation.
In plants, vascular cells are connected end to end to form vascular strands ().Citation1 In turn, vascular strands are connected with one another to form a network that extends throughout the plant. Through this vascular network, different organs and different parts of the same organ are connected with one another. In no organ, perhaps, is the sophistication of vascular connections more readily apparent than in the leaf. It is then no surprise that, just like all the expressions of pattern in nature, the patterns of vascular strands in plant leaves—the vein patterns—have captured the interest of artists and scientists since time immemorial. From a developmental standpoint, this interest seems justified, as veins form from within a population of seemingly identical cells.Citation2,Citation3 An additional, equally intriguing feature of vein patterning is that network topology changes in response to changes in growth conditions.Citation4-Citation7 What are the signals that control a complex process such as the formation of continuous veins and their connection into variable—yet functional—networks?
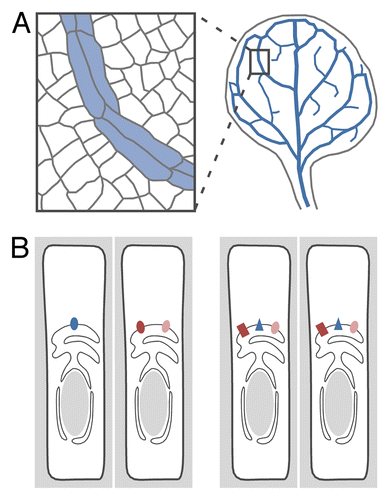
Figure 1. Control of vascular strand formation by intracellular auxin transport. (A) Plant vascular cells (blue fill) are connected end to end to form vascular strands—or “veins,” in the leaf (blue lines)—that are in turn connected to form a network. (B) Interaction in vein patterning between the genes encoding PIN5 (dark brown), PIN6 (blue), and PIN8 (light brown) could be accounted for by their expression in different vascular cells (left), their different vein-patterning functions (right; reflected in the different protein shapes), or combinations of the 2. See text for additional details.
Though a role for other molecules is by no means excluded, available evidence implicates indole-3-acetic acid (IAA)—the most abundant form of auxin in plantsCitation8—and its transport through plant tissues in vascular strand formation: application of IAA induces formation of vascular strands oriented away from the applied IAA and toward pre-existing strands;Citation9 the inductive effect of IAA on vascular differentiation is blocked by auxin transport inhibitors;Citation10 and auxin transport inhibitors induce vein patterning defects.Citation11,Citation12
In Arabidopsis, auxin is transported by members of at least 4 families: AUX1/LAX, ABCB/MDR/PGP, PILS, and PIN.Citation13-Citation20 The analysis of vein patterns in mutants of genes encoding members of these families is far from being exhaustive, but so far it has failed to suggest nonredundant vein-patterning functions for any single gene.Citation11,Citation14,Citation21 The only exception is PIN1, whose mutants have vein pattern defects that are similar to—though weaker than—those induced by auxin transport inhibitors.Citation11 This has suggested that PIN1 provides nonredundant functions in vein patterning but also that redundancy in vein patterning functions exists within the PIN family—and possibly other families—of auxin transporters.
Consistent with this observation, vein pattern defects of pin1 are synthetically enhanced by mutation in PIN6.Citation21 Because PIN1 is localized to the plasma membraneCitation22 and PIN6 to the endoplasmic reticulum (ER),Citation21 it is unlikely that PIN1 and PIN6 provide homologous functions in auxin transport; rather, PIN1 and PIN6 could act in distinct auxin-transport pathways with overlapping functions in vein patterning.
By contrast, the quantitative enhancement of vein pattern defects of pin1;pin6 (pin1;6 hereafter) double mutants by pin8Citation21 and the ER-localization of both PIN6Citation21 and PIN8Citation18,Citation19,Citation21 suggest that these 2 proteins provide redundant and homologous functions in PIN1-dependent vein patterning. Mutation in the gene encoding the ER-localized PIN5Citation17 fails to shift the spectrum of vein pattern phenotypes of pin1;6;8 toward more abnormal classes; instead, the phenotype spectrum of pin1;5;6;8 is similar to that of pin1;6.Citation21 As pin5 vein patterns are normal,Citation21 the partial normalization of pin1;6;8 defects by pin5 is unlikely to be the result of the sum of opposite effects of pin5 and pin8 on vein patterning; rather, it suggests that pin5 suppresses effects of pin8. This conclusion is also supported by the finding that pin5;8 pollen is normal even though pollen is equally defective in pin5 and pin8.Citation18 Though pin5 can suppress effects of pin8, it cannot suppress effects of pin6: the phenotype spectrum of pin1;5;6 is in fact no different from that of pin1;6.Citation21 Why can pin5 suppress the effects of pin8 but cannot suppress those of pin6?
One possibility is that PIN5 and PIN8 are expressed in the same cells but that PIN5 is not expressed in PIN6-expressing cells (, left). PIN5 is expressed in mature veins,Citation17 and PIN6 and PIN8 are expressed in developing veins;Citation21 however, whether expression of PIN5 overlaps with that of PIN6 or PIN8 is unknown. One other possibility is that PIN6 and PIN8 provide different (, right); however, this is inconsistent with the quantitative enhancement of pin1;6 defects by pin8. Further, expression of PIN6 or PIN8 by the same early-vascular promoter induces similar vein-patterning defects—defects that are opposite to those induced by expression of PIN5 by the same early vascular promoter.Citation21 This suggests that PIN6 and PIN8 can provide similar vein-patterning functions and that PIN5 can provide vein patterning functions opposite to those of either PIN6 or PIN8. Some morphological defects induced by PIN8 overexpression are normalized by PIN5 overexpression and enhanced by pin5.Citation18 It would be interesting to test whether vein pattern defects induced by expression of PIN8 or PIN6 by an early-vascular promoter are normalized by expression of PIN5 by the same promoter and enhanced by pin5.
Both pin6 and pin8 have normal vein patterns, but pin6;8 vein networks are more complex.Citation21 This suggests that PIN6 and PIN8 function redundantly in vein network formation, though the contributions of the 2 genes are likely unequal. In fact, only pin6 enhances vein pattern defects of pin1: pin8 does not.Citation21 What could account for this unequal behavior?
One possibility is that PIN6 and PIN8 have different vein-patterning functions (, right), but this is inconsistent with the appearance in pin6;8 of vein network defects that either single mutant lacks; it is also inconsistent with the quantitative enhancement of pin1;6 defects by pin8 and with the similar defects induced by expression of PIN6 or PIN8 by the same early-vascular promoter. One other possibility is that the unequal contribution of PIN6 and PIN8 to vein patterning results from their different expression (, left). PIN6 and PIN8 are both expressed during vein formation, but PIN6 expression is initiated at earlier stages.Citation21 Furthermore—as PIN3 and PIN7 in the rootCitation23—it is possible that PIN6 and PIN8 are expressed in distinct vascular cells. It would be interesting to test whether expression of PIN8 in the PIN6 domain reverts the spectrum of vein pattern phenotypes of pin1;6 to that of pin1.
The existence of intracellular auxin transport is a relatively recent discovery in the history of auxin transport research, and thus many questions still await answers. The vascular expression and function of all the intracellular auxin transporters of the PIN family suggest that vein patterning is one of the most informative processes to interrogate for these answers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Discovery Grants of the Natural Sciences and Engineering Research Council of Canada (NSERC). Sawchuk MG was supported by an NSERC CGS-M Scholarship and an NSERC CGS-D Scholarship.
References
- Esau K. Vascular differentiation in plants. New York, London: Holt, Rinehart, and Winston, 1965
- Foster AS. Foliar Venation in Angiosperms from an Ontogenetic Standpoint. Am J Bot 1952; 39:752 - 66; http://dx.doi.org/10.2307/2438624
- Pray TR. Foliar venation of Angiosperms. IV. Histogenesis of the venation of Hosta. Am J Bot 1955; 42:698 - 706; http://dx.doi.org/10.2307/2438644
- Sack L, Scoffoni C. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol 2013; 198:983 - 1000; http://dx.doi.org/10.1111/nph.12253; PMID: 23600478
- Scarpella E, Francis P, Berleth T. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development 2004; 131:3445 - 55; http://dx.doi.org/10.1242/dev.01182; PMID: 15226260
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. Evolution and function of leaf venation architecture: a review. Ann Bot (Lond) 2001; 87:553 - 66; http://dx.doi.org/10.1006/anbo.2001.1391
- Haritatos E, Medville R, Turgeon R. Minor vein structure and sugar transport in Arabidopsis thaliana.. Planta 2000; 211:105 - 11; http://dx.doi.org/10.1007/s004250000268; PMID: 10923710
- Ludwig-Müller J, Sass S, Sutter EG, Wodner M, Epstein E. Indole-3-Butyric Acid in Arabidopsis thaliana. 1. Identification and Quantification. Plant Growth Regul 1993; 13:179 - 7; http://dx.doi.org/10.1007/BF00024260
- Sachs T. The development of vascular networks during leaf development. Curr Top Plant Biochem Physiol 1989; 8:168 - 83
- Gersani M. The Induction of Differentiation of Organized Vessels in a Storage Organ. Ann Bot (Lond) 1987; 59:31 - 4
- Mattsson J, Sung ZR, Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development 1999; 126:2979 - 91; PMID: 10357941
- Sieburth LE. Auxin is required for leaf vein pattern in Arabidopsis.. Plant Physiol 1999; 121:1179 - 90; http://dx.doi.org/10.1104/pp.121.4.1179; PMID: 10594105
- Barbez E, Kubeš M, Rolčík J, Béziat C, Pěnčík A, Wang B, Rosquete MR, Zhu J, Dobrev PI, Lee Y, et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 2012; 485:119 - 22; http://dx.doi.org/10.1038/nature11001; PMID: 22504182
- Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, Casimiro I, Perry P, Syed A, et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 2012; 24:2874 - 85; http://dx.doi.org/10.1105/tpc.112.097766; PMID: 22773749
- Geisler M, Murphy AS. The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett 2006; 580:1094 - 102; http://dx.doi.org/10.1016/j.febslet.2005.11.054; PMID: 16359667
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 2006; 312:914 - 8; http://dx.doi.org/10.1126/science.1123542; PMID: 16601150
- Mravec J, Skůpa P, Bailly A, Hoyerová K, Krecek P, Bielach A, Petrásek J, Zhang J, Gaykova V, Stierhof YD, et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009; 459:1136 - 40; http://dx.doi.org/10.1038/nature08066; PMID: 19506555
- Ding Z, Wang B, Moreno I, Dupláková N, Simon S, Carraro N, Reemmer J, Pěnčík A, Chen X, Tejos R, et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis.. Nat Commun 2012; 3:941; http://dx.doi.org/10.1038/ncomms1941; PMID: 22760640
- Dal Bosco C, Dovzhenko A, Liu X, Woerner N, Rensch T, Eismann M, Eimer S, Hegermann J, Paponov IA, Ruperti B, et al. The endoplasmic reticulum localized PIN8 is a pollen-specific auxin carrier involved in intracellular auxin homeostasis. Plant J 2012; 71:860 - 70; http://dx.doi.org/10.1111/j.1365-313X.2012.05037.x; PMID: 22540348
- Chen R, Hilson P, Sedbrook J, Rosen E, Masson PH. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci U S A 1998; 95:15112 - 7; PMID: 9844024
- Sawchuk MG, Edgar A, Scarpella E. Patterning of leaf vein networks by convergent auxin transport pathways. PLoS Genet 2013; 9:e1003294; http://dx.doi.org/10.1371/journal.pgen.1003294; PMID: 23437008
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998; 282:2226 - 30; http://dx.doi.org/10.1126/science.282.5397.2226; PMID: 9856939
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen AP, Helariutta Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol 2011; 21:917 - 26; http://dx.doi.org/10.1016/j.cub.2011.04.017; PMID: 21620702