Abstract
Protein of nuclear encoded SUV3 (suppressor of Var 3) gene is a DNA and RNA helicase, localized in mitochondria and is a subunit of the degradosome complex involved in regulation of RNA surveillance and turnover. To overcome the abiotic stress-induced loss of crop yield, a multi-stress tolerant trait is required. Beside salinity stress the heavy metals including cadmium and zinc also affect the yield and quality of food crops. Since rice is a one of the staple food therefore it is important to develop a multi-stress including salinity and metal tolerant variety. Recently we have reported the role of OsSUV3 in salinity stress tolerance in rice; however, its role in metal stress has not been studied so far. Here we report that in response to cadmium and zinc stress the OsSUV3 transcript level is induced in rice and its overexpression in transgenic IR64 rice plants confers the metal stress tolerance. In addition to its previously reported role in salinity stress tolerance, this study further shows the role of OsSUV3 helicase in cadmium and zinc stress tolerance suggesting its involvement in multi-stress tolerance.
Abiotic stresses (such as high salinity, drought and flood, high and low temperatures) remain the greatest constraint to crop production. It affects growth productivity and triggers a series of, biochemical and molecular changes in plants which appears in the form of morphological and physiological changes in crops. Worldwide, it has been estimated that approximately 70% of yield reduction is the direct result of abiotic stresses.Citation1 Heavy metals have also become a critical environmental concern due to their potential adverse ecological effects. The regulatory limit of cadmium (Cd) in agricultural soil is 100 mg/kg soil.Citation2 This threshold is continuously exceeding because of several human activities. Plants exposed to high levels of Cd causes reduction in photosynthesis, water uptake, and nutrient uptake. Soil contaminated with zinc (Zn) may cause phytotoxicity when the concentrations of Zn found in contaminated soils exceed to those required as nutrients. High levels of Zn in soil inhibit many plant metabolic functions which resulted in retarded growth of both root and shoot.Citation3-Citation5 Rice is the staple food for about 50 per cent of the world’s population that resides in Asia, where 90 per cent of the world’s rice is grown and consumed. In Asia, India has the largest area under rice (41.66 million ha) accounting for 29.4 per cent of the global rice area. Of the total harvested area, about 46 per cent is irrigated with 28 per cent rain fed lowland, 12 per cent rain fed upland and 14 per cent flood prone. Rice is one of the major traded commodities in the world with a total quantity traded touching 16.4 million tonnes. The southeast countries account for about 40 per cent of the rice trade in the world.Citation6 Abiotic stress causes reduction in productivity of rice crops. Genetic modification in rice could be one of the effective way to develop stress-tolerant cultivars. Molecular techniques involve the development of genetically engineered plants by the introduction and/or overexpression of selected genes which can grow in abiotic stress conditions. In our previous studyCitation7 we have isolated and characterized OsSUV3 gene and reported that the encoded protein contains DNA and RNA helicase activity and functions in providing salinity stress tolerance by maintaining antioxidant machinery and enhancing photosynthesis in rice (Oryza sativa L. cv IR64). SUV3 helicase was initially reported in yeast (Saccharomyces cerevisiae) as a dominant suppressor allele. It plays an important role in the RNA surveillance system, regulates the stability of mature mRNAs, the removal of aberrantly formed mRNAs and the rapid degradation of non coding processing intermediates. In this study we report OsSUV3 provides first direct evidence of its function in imparting metal stress tolerance without reduction of biomass.
The 1.74 kb rice SUV3 (OsSUV3) gene has been cloned (accession number: GQ982584) and IR64 rice transgenic plants overexpressing this gene in sense and antisense orientations have been raised as described earlier.Citation7 As an ideal control the rice transgenic plants with empty vector (pCAMBIA1301) were also raised as the method described earlierCitation7 and named as vector control (VC) plant. For isolation of total RNA for quantitative real-time PCR (qRT-PCR) the wild-type (WT) rice (Oryza sativa cv IR 64) plants were grown in vermiculite in standard green house condition for 21 d with regular supplementation of Hoagland solution. The plants were allowed to grow in metal stress like CdCl2 (200 μM) and ZnCl2 (300 μM). Leaf samples were harvested at different time (1h, 2h, 3h, 6h and 12h) intervals. Isolation of total RNA and the expression analysis of OsSUV3 gene was performed by qRT-PCR according to method described earlierCitation8 using primers (forward 5′-CAG TTG AGA TGG CCG ACA-3′ and reverse 5′-CAG CTG GGT CAC CAC AAA-3′) and was normalized with α-tubulin primers (forward 5′-GGT GGA GGT GAT GAT GCT TT-3′ and reverse 5′-ACC ACG GGC AAA GTT GTT AG-3′). The qRT-PCR was repeated for three times independently for each time point. Relative gene expression was calculated using the 2-ΔΔCT values following Livak’s method.Citation9 For metal stress tolerance the leaf disk (~1cm × 1cm) from T1 SUV3 overexpressing transgenic rice (sense and antisense) and control plants (empty vector transformed [VC] and WT) were float in metal solutions (200 μM CdCl2 and 300 μM ZnCl2) for 72 h and total chlorophyll content were measured according to method described earlier.Citation10 For checking the post-germination growth in presence of metal stress the transgenic rice (OsSUV3) T2 seeds were grown on MS media containing predetermined concentration of CdCl2 (200 μM) and ZnCl2 (300 μM) in green house at 28 °C under 16 h light for 2 wk and their growths such as fresh biomass were observed.
A significant increase in the range of 2.4-fold to 6.3-fold was observed till 12 h in the expression level of OsSUV3 under the exposure to 200 μM CdCl2 treatment (). In the presence of 300 μM ZnCl2, the expression level was increased from 2.7-fold to 4.9-fold till 12 h. A slight decrease at 3 h was also observed (). Leaf disks from T1 sense transgenic lines were found more tolerant to both the metals (200 μM CdCl2 and 300 μM ZnCl2) as compared with WT, VC and AS plants after 72h (). Loss of chlorophyll was lesser in sense transgenic lines under the stress. Total chlorophyll content was significantly higher in transgenic (80%) as compared with WT, AS and VC plants after 200 μM CdCl2 and 300 μM ZnCl2 (). Seeds of T2 sense transgenic plants (L1-L3) showed good post-germination growth under 200 μM CdCl2 and 300 μM ZnCl2 stress while WT, AS and VC seeds showed much lesser germination under same conditions (). At 200 μM CdCl2 and 300 μM ZnCl2 a statistically significant growth difference was observed in between the WT, antisense, VC and transgenic lines. Under exposure to 200 μM CdCl2 and 300 μM ZnCl2 the fresh weight of transgenic lines were significantly increased (more than 70%) as compared with WT, AS and VC plants ().
Figure 1. Response of OsSUV3 transgenic plants to heavy metal stress. Quantitative real-time PCR analysis of OsSUV3 under different abiotic stress conditions (A) 200 µM CdCl2 (B) 300 µM ZnCl2. Total RNA isolation was done from leaf samples collected at different time intervals (viz. One h, 2 h, 3 h, 6 h, 12 h). Bars indicate the standard error (± SE) calculated from three independent experiments. (C) Leaf disk senescence assay of OsSUV3 transgenic rice plants along with antisense (AS), vector control (VC) and wild-type (WT) in presence of 200 µM CdCl2 and 300 µM ZnCl2. (D) Estimation of total chlorophyll content of OsSUV3 transgenic plants along with AS, VC and WT plants after CdCl2 stress. (E) Estimation of total chlorophyll content of OsSUV3 transgenic plants after ZnCl2 stress. (F) Germination test of T2 SUV3 transgenic AS, VC and WT seeds in MS plate supplemented with 200 µM CdCl2. (G) Germination of T2 SUV3 transgenic seeds after 300 µM ZnCl2. (H) Fresh weight per 10 seedlings from MS plate supplemented with 200 µM CdCl2. (I) Fresh weight per 10 seedlings from MS plate supplemented with 300 µM ZnCl2.
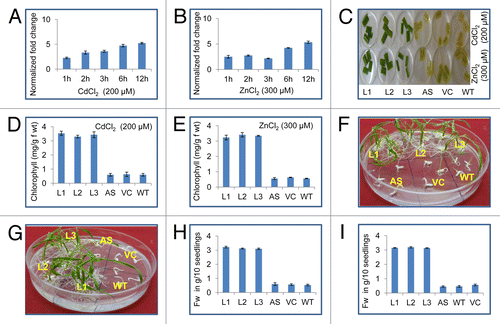
The expression of OsSUV3 transcript was upregulated in the presence of cadmium and zinc in rice seedlings. These observations suggest that OsSUV3 is involved in maintaining the homeostasis of these ions. Earlier it has been shown that in response to NaCl the transcript level of OsSUV3 gene was induced several folds.Citation7 Enhanced expression of OsSUV3 with respect to the metals stress suggests that it might be regulated through Cd2+ and Zn2+ dependent signal transduction pathways. The physiological parameters like fresh biomass of the plant are frequently used as a parameter to monitor the effects of heavy metal stress. Reduction of total fresh weight arises due to altered physiological phenomena in the presence of toxic levels of cadmium and zinc.Citation11 Reduction of chlorophyll in response to cadmium and zinc exposure indicates growth retardation and weakening of pigment biosynthetic pathway. It has been reported that Cd2+ induces depletion of chlorophyll content in a variety of plants.Citation12,Citation13 However, in this study transgenic rice plants overexpressing OsSUV3 possess significantly higher chlorophyll under Cd2+ stress. Hence, it appears that the transgenic rice plants are in a better state with respect to these pigments. This might be due to the possibility of OsSUV3 mediated Cd2+ ion efflux thereby maintaining the ion homeostasis for normal growth of exposed cells. Overall, this study indicates the novel role of OsSUV3 in metal (cadmium and zinc) stress tolerance. Previously its role in salinity stress tolerance has been shownCitation7 and now in metal stress tolerance (this study), suggesting future potential for using OsSUV3 in genetic approaches to improve plant performance and multi-stress tolerance.
| Abbreviations: | ||
| AS | = | antisense |
| Cd | = | cadmium |
| Os | = | Oryza sativa |
| SUV3 | = | suppressor of Var 3 |
| VC | = | vector control |
| WT | = | wild-type |
| Zn | = | Zinc |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Work on plant helicases and abiotic stress tolerance in NT’s laboratory is partially supported by Department of Biotechnology (DBT), Government of India.
References
- Acquaah G. Principles of plant genetics and breeding. Blackwell, Oxford, UK. 2007.
- Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 1995; 109:1427 - 33; PMID: 12228679
- Choi JM, Pak CH, Lee CW. Micronutrient toxicity in French marigold. J Plant Nutr 1996; 19:901 - 16; http://dx.doi.org/10.1080/01904169609365169
- Ebbs SD, Kochian LV. Toxicity of zinc and copper to Brassica species: implications for phytoremediation. J Environ Qual 1997; 26:776 - 81; http://dx.doi.org/10.2134/jeq1997.00472425002600030026x
- Fontes RLS, Cox FR. Zinc toxicity in soybean grown at high iron concentration in nutrient solution. J Plant Nutr 1998; 21:1723 - 30; http://dx.doi.org/10.1080/01904169809365517
- Rai M. International year of rice – An overview. Indian Farming 2004; 54:3 - 6
- Tuteja N, Sahoo RK, Garg B, Tuteja R. OsSUV3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR64). Plant J 2013; 76:115 - 27; http://dx.doi.org/10.1111/tpj.12277; PMID: 23808500
- Jayaraman A, Puranik S, Rai NK, Vidapu S, Sahu PP, Lata C, Prasad M. cDNA-AFLP analysis reveals differential gene expression in response to salt stress in foxtail millet (Setaria italica L.). Mol Biotechnol 2008; 40:241 - 51; http://dx.doi.org/10.1007/s12033-008-9081-4; PMID: 18592419
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402 - 8; http://dx.doi.org/10.1006/meth.2001.1262; PMID: 11846609
- Witham FH, Blaydes DF, Devlin RM. Experiments in Plant Physiology. Van Nostrand Reinhold Company, New York, 1971; pp: 55-56.
- Demirevska-Kepova K, Simova-Stoilova L, Stoyanova Z, Feller U. Cadmium stress in barley: growth, leaf pigment, and protein composition and detoxification of reactive oxygen species. J Plant Nutr 2006; 29:451 - 68; http://dx.doi.org/10.1080/01904160500524951
- Krantev A, Yordanova R, Janda T, Szalai G, Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 2008; 165:920 - 31; http://dx.doi.org/10.1016/j.jplph.2006.11.014; PMID: 17913285
- Liu D, Zou J, Wang M, Jiang W. Hexavalent chromium uptake and its effects on mineral uptake, antioxidant defence system and photosynthesis in Amaranthus viridis L. Bioresour Technol 2008; 99:2628 - 36; http://dx.doi.org/10.1016/j.biortech.2007.04.045; PMID: 17570658
