Abstract
RING (REALLY INTERESTING NEW GENE) proteins with E3 ligase activity are largely represented in plants. They have been shown to play important roles in the regulation of many biological processes by recognizing target proteins for ubiquitination. PtaRHE1, encoding a poplar RING-H2 domain-containing protein with E3 ligase activity has been previously shown to be expressed during the establishment of secondary vascular system in poplar. In the present report, we demonstrate that the expression of PtaRHE1 and the accumulation of its corresponding protein are modulated by the relative atmospheric and soil humidity and by abscisic acid. Overall, the integrated data are discussed within a working model highlighting a plausible function of PtaRHE1 in the signaling and/or in the regulation of water status in poplar.
PtaRHE1 was identified upon a screening aimed at determining regulatory genes involved in the establishment of secondary vascular system in Populus tremula x P. alba (clone INRA 717–1B4).Citation1PtaRHE1, encoding a RING-H2 protein of 293 amino acids, is structurally related to the Arabidopsis Tóxicos en Levadura (ATL) family.Citation2 In poplar stems, PtaRHE1 expression was localized in cambial zone, in adjacent xylem and phloem cells as well as in ray initials and derivatives, as investigated by in situ RT-PCR.Citation1 By histochemical β-glucuronidase (GUS) staining, a similar GUS expression pattern was observed in pPtaRHE1::GUS transgenic tobacco.Citation3 In addition, pPtaRHE1 was found to be induced in tobacco by the pathogens Agrobacterium tumefaciens (C58 strain), Rhodococcus fascians (D188 strain), and Pseudomonas syringae pv tabaci, as well as by elicitors such as salicylic acid and cellulase.Citation3 Various RING finger proteins have been found to be involved in the regulation of hormone signaling pathways such as the Arabidopsis BRH1 in brassinosteroid signaling,Citation4 SINAT5 in auxin response,Citation5 and SDIR1 in abscisic acid (ABA) signaling.Citation6 In the same way to what was postulated for several RING finger proteins, PtaRHE1 might be part of the overall signal involved in plant development and defense.Citation7
Most proteins containing a RING domain have been recognized as E3 ligase in ubiquitination processesCitation8 and in vitro auto-ubiquitination assay revealed that PtaRHE1 possesses E3 ligase activity in the presence of UbcH5a ubiquitin conjugating enzyme (UBC).Citation3 Therefore, PtaRHE1 is presumed to catalyze the transfer of a ubiquitin from an E2 UBC to a specific, but not yet identified, protein that will be targeted either for degradation, for translocation in a particular cell compartment or for protein conformational modifications.Citation3
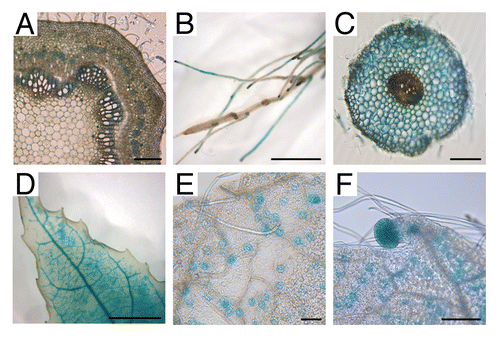
Here, we report on further functional analysis of PtaRHE1 in P. tremula x P. alba. An expression study was performed by histochemical GUS staining in different tissues and organs of 2-mo-old in vitro transgenic poplars carrying a pPtaRHE1::GUS construct. As shown in , pPtaRHE1 drives GUS expression mainly in stem phloem (including phloem fibers) and xylem tissues, in roots (both in vascular cylinder and in cortex cells), and in leaf veins, stomata, and hydathodes. Although pPtaRHE1 is developmentally regulated, the expression pattern in young plantlets suggests a link between pPtaRHE1 expression and anatomical structures involved in water conduction in plants.
Figure 1.pPtaRHE1 driven GUS expression in different organs and tissues of 2-mo-old in vitro P. tremula x P. alba. (A) cross section of stem; (B) roots; (C) cross section of root; (D) leaf; (E) close up of leaf showing stomata; (F) close up of leaf showing stomata and an hydathode. Scale bars represent 0.1 cm (A) and (C), 0.5 cm (B), 1 cm (D), and 100 µm (E) and (F).
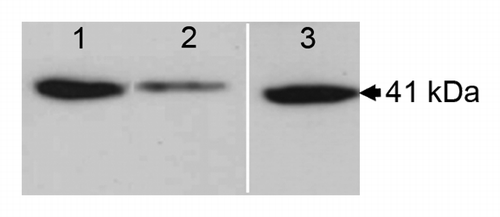
To unravel the possible function of PtaRHE1 in water conduction and/or in water status in poplar, 2-mo-old in vitro plants were analyzed either for GUS staining (pPtaRHE1::GUS line) or for protein accumulation by western blot (wild type plant) before and after 3 wk of acclimatization. As shown in , for in vitro plants (100% relative humidity), GUS staining () was correlated with the accumulation of PtaRHE1 protein in leaves (). Following acclimatization, both GUS staining and PtaRHE1 protein were no longer detected in leaves (). In these conditions, the root system is in approximately 100% relative humidity whereas the aerial part is in about 40% relative humidity (controlled phytotron condition). When these acclimatized plants were transferred to a mini-greenhouse for one wk, a situation close to the in vitro condition with approximately 100% relative soil and atmospheric humidity, both GUS staining () and PtaRHE1 protein accumulation () resumed. We can therefore conclude that in leaves: 1) PtaRHE1 expression is regulated at the transcriptional level, 2) the molecular weight of the cross-reacting protein (41 kDa) corresponds probably to the accumulation of a conjugated form of PtaRHE1 (33 kDa) to one ubiquitin (8.5 kDa) (in agreement with the reported in vitro mono-ubiquitination of recombinant PtaRHE1),Citation3 and 3) PtaRHE1 accumulation in leaves is correlated with the relative atmospheric humidity, being present when the humidity is high (almost 100%) and downregulated in low air humidity (40%).
Figure 2. PtaRHE1 response to atmospheric humidity. (A)-(C) GUS expression in leaves of pPtaRHE1-GUS transgenic P. tremula x P. alba, as determined by histochemical GUS assay. Scale bars represent 1 cm. (D) western blot analysis of total protein extracts (15 µg) from leaves of P. tremula x P. alba. (A) and (D) - lane 1, 2-mo-old in vitro plant. (B) and (D) – lane 2, plant acclimatized for 3 wk. (C) and (D) – lane 3, acclimatized plant transferred to a mini-greenhouse for 1 wk. Lane 3 corresponds to a different western blot analysis than lanes 1 and 2.

To further document these conclusions, PtaRHE1 accumulation was evaluated in stem xylem tissue in different conditions of relative air and soil humidity (). PtaRHE1 was found to accumulate in 3-mo-old acclimatized poplar plants watered 3 times a wk (air humidity 40%, soil humidity 100%) (). PtaRHE1 accumulation decreased when these plants were not watered for one wk (air humidity 40%, soil humidity 40%) () but resumed when these plants were watered again 3 times a wk during one wk (). Hence, by reducing water availability in soil, PtaRHE1 accumulation in xylem tissues decreased.
Figure 3. PtaRHE1 accumulation in stem xylem of P. tremula x P. alba in response to water availability. Western blot analysis was performed with total protein extract (15 µg) from xylem of 5-mo-old poplar. Plants watered 3 times a wk (lane 1), plants not watered for 7 d (lane 2), and plant re-watered for 7 d (lane 3). Lane 3 corresponds to a different western blot analysis than lanes 1 and 2.
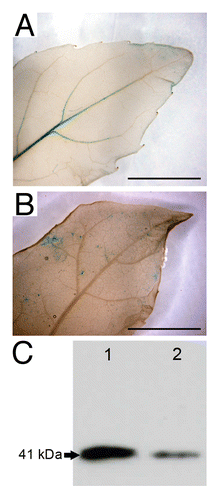
To cope with water deficit, plants have developed a root to shoot signaling cascade where ABA biosynthesis is induced in the root system.Citation9 ABA is transported through the xylem to the leaves where it is thought to regulate stomatal closure, thereby reducing transpiration and preventing decline in water potential.Citation9,Citation10 As shown in , when in vitro pPtaRHE1::GUS poplars were transferred in ABA-supplemented medium (150 µM) for 6 h, GUS staining was no longer detectable in leaf veins (), as compared with plant transferred to ABA-free medium (), and the accumulation of the protein decreased in xylem tissue (), suggesting that ABA inhibits PtaRHE1 expression. Similar results were reported for the Arabidopsis AtATL78, encoding a RING-H2 E3 ligase, which is downregulated by ABA.Citation11 The pPtaRHE1 used in this study includes a 1207 bp long sequence upstream of the ATG codon that contains several potential cis-acting elements including 2 ABA-responsive elements (ABREs) (core ACGTG)Citation3 and 2 ABA–repression motifs (CAAGTTG), as identified by Wang et al.Citation12
Figure 4.PtaRHE1 response to ABA treatment in 2-mo-old in vitro poplar plants. (A) Leaf from pPtaRHE1::GUS plant transferred in ABA-free liquid medium for 6 h; (B) Leaf from pPtaRHE1::GUS plant transferred in ABA-supplemented medium (150 µM) for 6 h. Scale bars represent 1 cm; (C) western blot analysis of total protein extract (15 µg) from leaves of P. tremula x P. alba incubated in ABA-free liquid medium (lane 1) or in ABA-supplemented medium (lane 2).
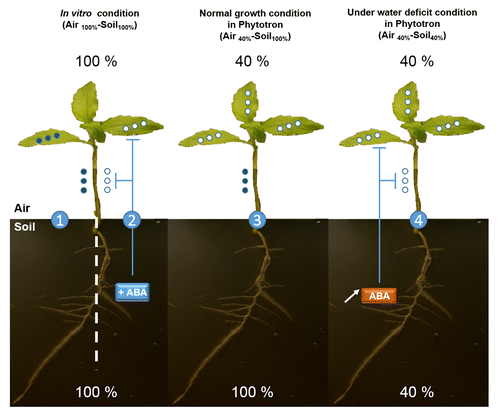
By compiling the overall described data, a speculative model for PtaRHE1 function, modulated by the root to shoot ABA signaling, is suggested in . According to the relative humidity in air and in soil, 3 different conditions can be distinguished. The first condition corresponds to plant growing in vitro (100% relative humidity) where PtaRHE1 accumulated in both leaves and xylem tissue. PtaRHE1 may, directly or indirectly, contribute to the evacuation of the water excess through, for instance, stomata and hydathodes present in leaves of plants. The second condition corresponds to in vitro plants cultured in ABA-supplemented medium where both PtaRHE1 transcript and protein accumulation are inhibited in leaves and in xylem tissue. This expression pattern replicates the effect observed in plants cultivated in phytotron and submitted to water deficit condition (air and soil: 40% relative humidity). The third condition corresponds to plants cultivated in standard conditions for growth (air 40% humidity – soil 100% humidity). In this case, PtaRHE1 is detected in xylem tissue but not in leaves, suggesting that PtaRHE1 has a tissue specific expression pattern, which is conditioned by environmental factors such as, for instance, the relative humidity. Several RING-H2 E3 ligases have been described to respond to water deficit throughout ABA signaling. For instance, upregulation of XERICO, encoding an Arabidopsis RING-H2 protein, induces a dramatic increase in cellular ABA level and consequently drought tolerance,Citation13 RHA2a and RHA2b were shown to positively regulate ABA signalingCitation14 and, more recently, the maize ZmRFP1 was shown to respond to drought stress in an ABA-depending manner.Citation15
Figure 5. PtaRHE1 accumulation in poplar is affected by relative atmospheric and soil humidity as well as by ABA. (1) Under in vitro condition (air 100% - soil 100% relative humidity), PtaRHE1 is present in both leaves and stem. (2) When these plants were transferred to ABA-supplemented medium, PtaRHE1 was no longer detected. (3) In normal growth condition (air 40% - soil 100% relative humidity), PtaRHE1 accumulated in stem but not in leaves. (4) When acclimatized plants were not watered for one week (air 40% - soil 40% relative humidity), PtaRHE1 accumulation decreased in both leaves and stem xylem. It is suggested that under water deficit condition, ABA synthesized in roots affects PtaRHE1 expression in both stem and leaves. Open circles: PtaRHE1 is not accumulated; closed circles: PtaRHE1 is accumulated.
Interestingly enough, the pPtaRHE1 expression pattern in poplar stem is similar to that reported by in situ mRNA hybridization for the genes encoding the water channel intrinsic proteins PIP2:3 and PIP2:5 and the tonoplast intrinsic protein TIP2:1.16According to this study, these aquaporins (AQPs) are expressed in cambial region, phloem, xylem, and rays in poplar stems. As suggested by Hacke et al.,Citation17 the expression of AQPs in rays may increase radial flow of water from xylem and phloem to the cambial region where AQPs may help sustain rapid cell division and expansion of developing vessel elements.
Therefore, a plausible direct or indirect link between AQPs and PtaRHE1 can be suggested. PtaRHE1, as an E3 ligase, is probably recruited for the regulation of the activity and/or the degradation of target protein(s). Although the precise target protein for PtaRHE1 is not yet known, one can assume that PtaRHE1 contributes to the control of water conduction by the degradation/modulation of protein(s) either involved in the signaling cascade or in the transcriptional regulation of gene(s) coding for protein(s) playing a direct role in water conduction (for instance, AQPs). Along the same line, water stress experiments, made in poplar, have shown an increase in PIP content in root, stem, and leaf of water stressed plants.Citation16,Citation18,Citation19
To better decipher the potential role of PtaRHE1 in ABA signaling, further experiments are required: 1) to establish the relationship between ABA homeostasis, the occurrence of PtaRHE1 as well as AQPs in specific tissues and/or organs, 2) to identify the target protein(s) of PtaRHE1 as an E3 ligase, and 3) to characterize PtaRHE1 overexpressing and downregulated transgenic poplar lines under drought stress conditions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Moussawi J is a scholar of the Lebanese National Council for Scientific Research (CNRS-L) and received support from the “Fonds Alice et David Van Buuren.” Baucher M is Senior Research Associate of the FRS-FNRS (Belgian Fund for Scientific Research). Baldacci-Cresp F received a grant post-doc IN from the Bureau des Relations Internationales et de la Coopération of Université Libre de Bruxelles. This study was supported by FRS-FNRS grant “Crédit aux chercheurs 1.5107.12,” by the bilateral France-Wallonie-Bruxelles Tournesol program and by COST Action FP0905.
References
- van Raemdonck D, Pesquet E, Cloquet S, Beeckman H, Boerjan W, Goffner D, El Jaziri M, Baucher M. Molecular changes associated with the setting up of secondary growth in aspen. J Exp Bot 2005; 56:2211 - 27; http://dx.doi.org/10.1093/jxb/eri221; PMID: 15996985
- Aguilar-Hernández V, Aguilar-Henonin L, Guzmán P. Diversity in the architecture of ATLs, a family of plant ubiquitin-ligases, leads to recognition and targeting of substrates in different cellular environments. PLoS One 2011; 6:e23934; http://dx.doi.org/10.1371/journal.pone.0023934; PMID: 21887349
- Mukoko Bopopi J, Vandeputte OM, Himanen K, Mol A, Vaessen Q, El Jaziri M, Baucher M. Ectopic expression of PtaRHE1, encoding a poplar RING-H2 protein with E3 ligase activity, alters plant development and induces defence-related responses. J Exp Bot 2010; 61:297 - 310; http://dx.doi.org/10.1093/jxb/erp305; PMID: 19892745
- Molnár G, Bancoş S, Nagy F, Szekeres M. Characterisation of BRH1, a brassinosteroid-responsive RING-H2 gene from Arabidopsis thaliana. Planta 2002; 215:127 - 33; http://dx.doi.org/10.1007/s00425-001-0723-z; PMID: 12012249
- Xie Q, Guo H-S, Dallman G, Fang S, Weissman AM, Chua N-H. SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 2002; 419:167 - 70; http://dx.doi.org/10.1038/nature00998; PMID: 12226665
- Zhang Y, Yang C, Li Y, Zheng N, Chen H, Zhao Q, Gao T, Guo H, Xie Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 2007; 19:1912 - 29; http://dx.doi.org/10.1105/tpc.106.048488; PMID: 17573536
- Schwechheimer C, Willige BC, Zourelidou M, Dohmann EMN. Examining protein stability and its relevance for plant growth and development. Methods Mol Biol 2009; 479:147 - 71; http://dx.doi.org/10.1007/978-1-59745-289-2_10; PMID: 19083189
- Deshaies RJ, Joazeiro CAP. RING domain E3 ubiquitin ligases. Annu Rev Biochem 2009; 78:399 - 434; http://dx.doi.org/10.1146/annurev.biochem.78.101807.093809; PMID: 19489725
- Finkelstein R.. Abscisic Acid Synthesis and Response. The Arabidopsis Book 2013; 11:e0166
- Zhang J, Davies WJ. Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant Cell Environ 1989; 12:73 - 81; http://dx.doi.org/10.1111/j.1365-3040.1989.tb01918.x
- Kim SJ, Kim WT. Suppression of Arabidopsis RING E3 ubiquitin ligase AtATL78 increases tolerance to cold stress and decreases tolerance to drought stress. FEBS Lett 2013; 587:2584 - 90; http://dx.doi.org/10.1016/j.febslet.2013.06.038; PMID: 23831064
- Wang R-S, Pandey S, Li S, Gookin TE, Zhao Z, Albert R, Assmann SM. Common and unique elements of the ABA-regulated transcriptome of Arabidopsis guard cells. BMC Genomics 2011; 12:216; http://dx.doi.org/10.1186/1471-2164-12-216; PMID: 21554708
- Ko J-H, Yang SH, Han K-H. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J 2006; 47:343 - 55; http://dx.doi.org/10.1111/j.1365-313X.2006.02782.x; PMID: 16792696
- Li H, Jiang H, Bu Q, Zhao Q, Sun J, Xie Q, Li C. The Arabidopsis RING finger E3 ligase RHA2b acts additively with RHA2a in regulating abscisic acid signaling and drought response. Plant Physiol 2011; 156:550 - 63; http://dx.doi.org/10.1104/pp.111.176214; PMID: 21478367
- Xia Z, Liu Q, Wu J, Ding J. ZmRFP1, the putative ortholog of SDIR1, encodes a RING-H2 E3 ubiquitin ligase and responds to drought stress in an ABA-dependent manner in maize. Gene 2012; 495:146 - 53; http://dx.doi.org/10.1016/j.gene.2011.12.028; PMID: 22245611
- Almeida-Rodriguez AM, Hacke UG. Cellular localization of aquaporin mRNA in hybrid poplar stems. Am J Bot 2012; 99:1249 - 54; http://dx.doi.org/10.3732/ajb.1200088; PMID: 22763351
- Hacke UG, Plavcová L, Almeida-Rodriguez A, King-Jones S, Zhou W, Cooke JEK. Influence of nitrogen fertilization on xylem traits and aquaporin expression in stems of hybrid poplar. Tree Physiol 2010; 30:1016 - 25; http://dx.doi.org/10.1093/treephys/tpq058; PMID: 20610665
- Secchi F, Zwieniecki MA. Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant Cell Environ 2010; 33:1285 - 97; http://dx.doi.org/10.1111/j.1365-3040.2010.02147.x; PMID: 20302602
- Leng H, Lu M, Wan X. Variation in embolism occurrence and repair along the stem in drought-stressed and re-watered seedlings of a poplar clone. Physiol Plant 2013; 147:329 - 39; http://dx.doi.org/10.1111/j.1399-3054.2012.01665.x; PMID: 22686493
