Abstract
Cellular auxin homeostasis controls many aspects of plant growth, organogenesis and development. The existence of intracellular auxin transport mediated by endoplasmic reticulum (ER)-localized PIN5, PIN6 and PIN8 proteins is a relatively recent discovery shaping a new era in understanding auxin-mediated growth processes. Here we summarize the importance of PIN6 in mediating intracellular auxin transport during root formation, leaf vein patterning and nectary production. While, it was previously shown that PIN6 was strongly expressed in rosette leaf cell types important in vein formation, here we demonstrate by use a PIN6 promoter-reporter fusion, that PIN6 is also preferentially expressed in the vasculature of the primary root, cotyledons, cauline leaves, floral stem, sepals and the main transmitting tract of the reproductive silique. The strong, vein- specific reporter gene expression patterns enabled by the PIN6 promoter emphasizes that transcriptional control is likely to be a major regulator of PIN6 protein levels, during vasculature formation, and supports the need for ER-localized PIN proteins in selecting specialized cells for vascular function in land plants.
Keywords: :
PIN Mediated Polar Auxin Transport defines Plant Development
The dynamic and differential regulation of plant signaling molecules orchestrates a range of plant growth and developmental responses via intrinsic and environmental cues. The plant hormone, auxin, is an essential regulatory molecule affecting growth processes such as morphogenesis, embryogenesis, organogenesis, tropism, flower development and leaf venation as well as root formation. The function of auxin in these processes has been extensively reviewed.Citation1-Citation5 Auxin transport is mediated by a specific set of protein carriers, where AUX1 (Auxin Resistant 1) and LAX (Like AUX1) act as auxin influx carriers, while PIN (Pin-formed) and members of the multidrug resistance/P-glycoprotein family (ABCB/MDR/PGP) play a role as auxin efflux carriers.Citation6-Citation13 The Arabidopsis PIN protein family provides essential transport machinery to control auxin efflux and exhibit molecular divergence in their localization to either the plasma membrane (PM) (e.g., PIN1, 2, 3, 4, 7) or endoplasmic reticulum (ER) (e.g., PIN 5, 6, 8). Within last two decades the characterization of these PIN proteins demonstrated their unique and synergistic functions during plant development by controlling the intercellular and intracellular auxin transport, thereby affecting distribution and the regulation of cellular auxin homeostasis.Citation7,Citation14-Citation21 Individual PIN proteins act redundantly given that single pin mutants can grow to complete a reproductive plant life cycle. The exception is PIN1, which is the major non-redundant PM localized PIN protein. A concerted action of PIN1, 2, 4 and 7 is required for essential directional polar auxin transport during embryogenesis and organogenesis.Citation22-Citation24 PIN3 in conjunction with other PIN proteins is involved in hypocotyl and root tropism and required for proper gravitropism.Citation16 The ER- localized PIN5, 6 and 8 proteins are involved in intracellular auxin distribution and homeostasis, where PIN5 and PIN8 are known to negatively regulate each other’s transport capability, affecting pollen development and function.Citation19,Citation21,Citation25,Citation26 The function of PIN6 as an auxin transporter, however, remained less obvious until very recent studies discovered essential roles for PIN6 in contributing to intracellular auxin homeostasis during root development,Citation27 reproductive nectar secretionCitation28 and leaf venation.Citation29
PIN6 is Required for Proper Auxin Response during Root Development and Nectar Secretion in Arabidopsis
We recently showed that the expression of PIN6 is developmentally regulated exhibiting cell-type-, tissue- and organ-specific expression patterns and affecting auxin-dependent root growth and reproductive development.Citation27 We demonstrated that the loss-of-function in pin6 mutants slightly reduced primary root length, and the number of lateral root primordia increased during later stages of root development revealing a function for PIN6 during root development.Citation27 These phenotypes observed in pin6 mutants were consistent with the cell-type and tissue-specific expression patterns observed in the root tip and lateral root primordia. Weak and variable expression was occasionally observed in the primary rootCitation27 and here we further show PIN6 specific expression in the root vasculature (), which could occur as a result of auxin-mediated induction of PIN6 gene expression.
Figure 1.PIN6 reporter expression is stronger in the vasculature of roots, cotyledons, leaves, stems and reproductive organs. GUS staining patterns of a representative transgenic line harboring PIN6 promoter (-1794bp) fused to β-glucuronidase (GUS).Citation27 Stronger GUS staining was observed in the vasculature of most tissues tested. Reporter gene expression was most intense in the shoot apex of 7 d old seedlings (A), vasculature of the floral stem (B and C), cauline leaves (D), floral sepals (E) and the silique (F), as well as in the nectary, pollen and floral organ boundaries (F). Plant tissues were incubated in a previously described GUS histochemical assay mixture for 24 h to enhance detection of GUS reporter gene activity.Citation36 Construction of the promoter-reporter binary vector (pMDC32-PIN6::GUS) and plant transformation were as previously described.Citation27
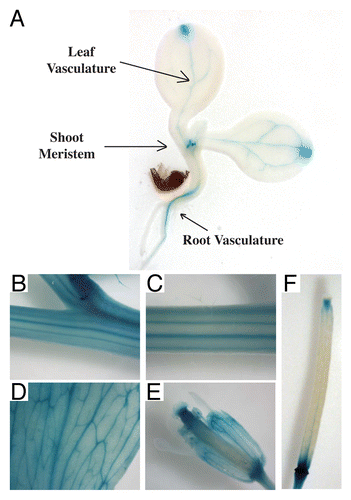
Another recent study showed that PIN6 and auxin regulate nectary production in Arabidopsis.Citation28 Here the authors showed that PIN6 is required for a proper auxin response as pin6 mutants displayed an abnormal floral and nectary phenotype, exhibiting petals that fail to fully expand and significantly smaller nectaries (stamens) with reduced nectary production. Clearly, auxin is an important factor in the regulation of nectar production and the maturation of lateral nectarines.Citation28 Our bioinformatics and promoter:reporter gene expression studies previously demonstrate that PIN6 is expressed in the nectaries and floral organ boundaries of the anthers (),Citation27 which is consistent with a newly discovered function for PIN6 in nectary production and intracellular auxin transport in pollen.Citation25,Citation28
Mis-Expression of PIN6 perturbs Auxin-Mediated Plant Development
The mis-expression of PIN6 can alter auxin transport and interfere with auxin homeostasis in developmental and growth processes (e.g., shoot apical dominance, rosette morphology, lateral root primordia development, adventitious root formation, root hair outgrowth and root waving).Citation27 Contrary to pin6 mutants, auxin homeostasis and auxin related growth processes in the roots were affected by PIN6 overexpression, which resulted in a clear reduction in the number of lateral root primordia.Citation27 The more striking pronounced root waving phenotype, lack of root hairs and enhanced adventitious root formation emphasize that regulatory control over PIN6 expression is likely to be an important molecular mechanism in root growth processes. Indeed, the changes in root morphology as a result of PIN6 mis-expression are supported by our reporter gene analysis showing PIN6 expression in the root tip, cells of lateral root primordia,Citation27 and more erratically in the root vasculature (). Auxin response elements were found in the PIN6 promoter and indeed PIN6 gene expression is inducible by auxin.Citation27 However, given the low abundance of PIN6 expression in most tissues and that the PIN6 promoter region appears to be strongly marked with the repressive chromatin modification mark of histone-3 lysine-27 trimethylation (http://www.mcdb.ucla.edu/Research/Jacobsen/),Citation27,Citation30 it is interesting to speculate that PIN6 is tightly regulated in order to avoid mis-expression that leads to the above root phenotypes.Citation27
Above the ground, PIN6 overexpression resulted in stunted growth and reduced apical dominance. For instance, PIN6 mis-expression dramatically altered rosette development, forming a more compact rosette with a reduced leaf area and shorter petioles, and affected apical dominance.Citation27 Even though these morphological changes were unexpected they are consistent with auxin regulated growth processes. The increased chlorophyll content relative to leaf fresh weight was perplexing and this may arise from an increased number of leaf mesophyll cells or chloroplasts per cell. While there are reports suggesting that the regulation, synthesis and distribution of auxin can be closely associated with light regulated growth and development (including photomorphogenesis, chlorophyll production, and phototrophic responseCitation31-Citation35), a direct function for polar auxin transporters in controlling chlorophyll pigment production has yet to be reported. The enhanced chlorophyll phenotype arising from the overexpression of PIN6 warrants further investigation.
During reproduction, PIN6 overexpression enhanced floral stem thickness, reduced floral stem height and increased the number of reproductive siliques. Consistent with the changes in morphology, stronger PIN6 expression was observed in the shoot meristem, organ boundaries of the reproductive silique and floral stem.Citation27 An isolated inflorescence stem internode auxin transport assay demonstrated that PIN6 mis-expression decreased the total amount of polar auxin transported through the floral stem, which we hypothesized could explain the observed defects in apical dominance. Notably, PIN6-reporter expression was very strong in the floral stem, comparable to the CaMV35s promoter,Citation27 and therefore PIN6 must be essential in the floral stem tissues. Here we further demonstrate a vascular specific expression of PIN6 promoter-reporter activity in the primary floral stem () that was not previously reported. The phenotypes arising from PIN6 overexpression correlate strongly with the sites of PIN6 expression and support a function for PIN6 in controlling intracellular auxin transport within the floral stem vascular transport system.
Synergistic Interactions between PIN6 and Other PIN Proteins alter Patterning of Leaf Vein Networks
Recently it was shown that vein pattern formation in the Arabidopsis leaf was mediated by distinct and convergent auxin-transport pathways.Citation29 Perturbations in intercellular transport of auxin in pin1 mutant leaves showed mild defects in vein patterning. The loss-of-function pin6 mutants do not affect vein patterning; however, a pin1pin6 double mutant enhanced pin1 vein-pattern defects.Citation29 The authors next showed that intracellular PIN6-mediated auxin transport increases the levels of auxin-responsive PIN1 expression during vein formation.Citation29
Consistent with these observations, a translational fusion of PIN6 to GFP driven by the PIN6 promoter displayed expression in broad subepidermal domains that narrowed to sites associated with vein formation. The authors also demonstrated the ER-localization of PIN6 during vein development.Citation29 In fact, the network of leaf vein formation was shown to be regulated by convergent action of intercellular and intracellular auxin transport mechanism mediated through plasma membrane-localized PIN1 and ER localized PIN6, PIN8 and PIN5 proteins, respectively.Citation29
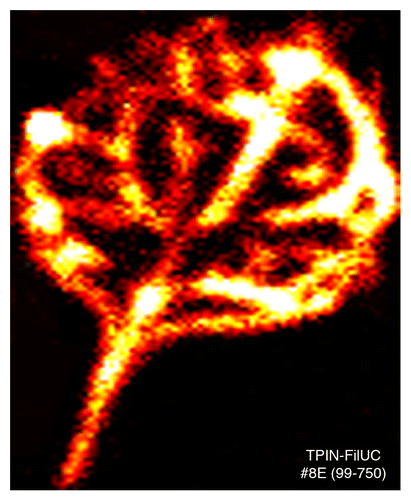
We confirm that our PIN6 promoter-reporter transgenic lines (pMDCPIN6::GUS and pPIN6:FiLUC)Citation27 enable strong expression (e.g., GUS staining pattern and luminescence) in the leaf vasculature ( and ). Next we showed that PIN6 expression was also observed in the vasculature of the primary root, cotyledons, cauline leaves, floral stem, sepals and the main transmitting tract of the silique (). The strong vein specific expression pattern of PIN6 and the demonstrated function for PIN6 in vein network formation, solidify the importance of auxin in regulating the patterning and formation of vein-like structures in land plants.
Figure 2.PIN6 is strongly expressed during leaf venation. An in vivo bioluminescence assay of a leaf from a 9 d old representative transgenic line harboring a PIN6::luciferase reporter fusion. Image J software was used to create false color image: the threshold was adjusted such that black color indicates no luminescence, red and yellow color reflect low to medium levels of luminescence respectively, whereas, white reflects higher levels of luminescence. Luciferase imaging was performed for 10 min using a previously described method.Citation36 Construction of the promoter-reporter binary vector (pTPIN6::FiLUC#8E) and plant transformation were as previously described.Citation27
Summary
PIN6 is expressed in specific cell and tissue types, depending upon growth conditions and developmental stages. PIN6 expression can be induced by auxin and may be controlled by repressive chromatin modification. PIN6 transcriptional expression patterns can be closely associated to phenotypic outcomes such as vein patterning, root development and nectary production that arise through mis-expression or loss-of-function in pin6. The recent advances in understanding the function of PIN6 further our knowledge of how intracellular PIN proteins modify overall auxin homeostasis and transport to define plant growth and development.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
CIC and BJP planned the research. CIC and NN performed experiments, prepared figures and wrote the short communication. All authors contributed to editing the manuscript.
Acknowledgments
We would like to thank Ulrike Mathesius and Kai Chan (ANU) for critical reading. The research was supported by the Australian Research Council (ARC) Centre of Excellence in Plant Energy Biology (CE0561495).
References
- Friml J. Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur J Cell Biol 2010; 89:231 - 5; http://dx.doi.org/10.1016/j.ejcb.2009.11.003; PMID: 19944476
- Korasick DA, Enders TA, Strader LC. Auxin biosynthesis and storage forms. J Exp Bot 2013; 64:2541 - 55; http://dx.doi.org/10.1093/jxb/ert080; PMID: 23580748
- Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 2010; 61:49 - 64; http://dx.doi.org/10.1146/annurev-arplant-042809-112308; PMID: 20192736
- Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. J Exp Bot 2012; 63:2853 - 72; http://dx.doi.org/10.1093/jxb/ers091; PMID: 22447967
- Sauer M, Robert S, Kleine-Vehn J. Auxin: simply complicated. J Exp Bot 2013; 64:2565 - 77; http://dx.doi.org/10.1093/jxb/ert139; PMID: 23669571
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 2005; 44:179 - 94; http://dx.doi.org/10.1111/j.1365-313X.2005.02519.x; PMID: 16212599
- Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, Wisniewska J, Tadele Z, Kubes M, Covanová M, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 2006; 312:914 - 8; http://dx.doi.org/10.1126/science.1123542; PMID: 16601150
- Löfke C, Luschnig C, Kleine-Vehn J. Posttranslational modification and trafficking of PIN auxin efflux carriers. Mech Dev 2013; 130:82 - 94; http://dx.doi.org/10.1016/j.mod.2012.02.003; PMID: 22425600
- Barbez E, Kleine-Vehn J. Divide Et Impera--cellular auxin compartmentalization. Curr Opin Plant Biol 2013; 16:78 - 84; http://dx.doi.org/10.1016/j.pbi.2012.10.005; PMID: 23200033
- Grunewald W, Friml J. The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J 2010; 29:2700 - 14; http://dx.doi.org/10.1038/emboj.2010.181; PMID: 20717140
- Wabnik K, Govaerts W, Friml J, Kleine-Vehn J. Feedback models for polarized auxin transport: an emerging trend. Mol Biosyst 2011; 7:2352 - 9; http://dx.doi.org/10.1039/c1mb05109a; PMID: 21660355
- Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 2001; 13:2441 - 54; PMID: 11701880
- Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, et al. Plant ABC proteins--a unified nomenclature and updated inventory. Trends Plant Sci 2008; 13:151 - 9; http://dx.doi.org/10.1016/j.tplants.2008.02.001; PMID: 18299247
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005; 433:39 - 44; http://dx.doi.org/10.1038/nature03184; PMID: 15635403
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 2002; a 108:661 - 73; http://dx.doi.org/10.1016/S0092-8674(02)00656-6; PMID: 11893337
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002; c 415:806 - 9; http://dx.doi.org/10.1038/415806a; PMID: 11845211
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 1998; 12:2175 - 87; http://dx.doi.org/10.1101/gad.12.14.2175; PMID: 9679062
- Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev 2006; 20:1015 - 27; http://dx.doi.org/10.1101/gad.1402406; PMID: 16618807
- Mravec J, Skůpa P, Bailly A, Hoyerová K, Krecek P, Bielach A, Petrásek J, Zhang J, Gaykova V, Stierhof YD, et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009; 459:1136 - 40; http://dx.doi.org/10.1038/nature08066; PMID: 19506555
- Viaene T, Delwiche CF, Rensing SA, Friml J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci 2013; 18:5 - 10; http://dx.doi.org/10.1016/j.tplants.2012.08.009; PMID: 22981345
- Ding Z, Wang B, Moreno I, Dupláková N, Simon S, Carraro N, Reemmer J, Pěnčík A, Chen X, Tejos R, et al. ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat Commun 2012; 3:941; http://dx.doi.org/10.1038/ncomms1941; PMID: 22760640
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003; 115:591 - 602; http://dx.doi.org/10.1016/S0092-8674(03)00924-3; PMID: 14651850
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 2003; b 426:147 - 53; http://dx.doi.org/10.1038/nature02085; PMID: 14614497
- Reinhardt D, Pesce E-R, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature 2003; 426:255 - 60; http://dx.doi.org/10.1038/nature02081; PMID: 14628043
- Dal Bosco C, Dovzhenko A, Palme K. Intracellular auxin transport in pollen: PIN8, PIN5 and PILS5. Plant Signal Behav 2012; 7:1504 - 5; http://dx.doi.org/10.4161/psb.21953; PMID: 22990451
- Dal Bosco C, Dovzhenko A, Liu X, Woerner N, Rensch T, Eismann M, Eimer S, Hegermann J, Paponov IA, Ruperti B, et al. The endoplasmic reticulum localized PIN8 is a pollen-specific auxin carrier involved in intracellular auxin homeostasis. Plant J 2012; 71:860 - 70; http://dx.doi.org/10.1111/j.1365-313X.2012.05037.x; PMID: 22540348
- Cazzonelli CI, Vanstraelen M, Simon S, Yin K, Carron-Arthur A, Nisar N, Tarle G, Cuttriss AJ, Searle IR, Benkova E, et al. Role of the Arabidopsis PIN6 auxin transporter in auxin homeostasis and auxin-mediated development. PLoS One 2013; 8:e70069; http://dx.doi.org/10.1371/journal.pone.0070069; PMID: 23922907
- Bender RL, Fekete ML, Klinkenberg PM, Hampton M, Bauer B, Malecha M, Lindgren K, A Maki J, Perera MA, Nikolau BJ, et al. PIN6 is required for nectary auxin response and short stamen development. Plant J 2013; 74:893 - 904; http://dx.doi.org/10.1111/tpj.12184; PMID: 23551385
- Sawchuk MG, Edgar A, Scarpella E. Patterning of leaf vein networks by convergent auxin transport pathways. PLoS Genet 2013; 9:e1003294; http://dx.doi.org/10.1371/journal.pgen.1003294; PMID: 23437008
- Zhang X, Clarenz O, Cokus S, Bernatavichute YV, Pellegrini M, Goodrich J, Jacobsen SE. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol 2007; 5:e129; http://dx.doi.org/10.1371/journal.pbio.0050129; PMID: 17439305
- Friml J, Palme K. Polar auxin transport--old questions and new concepts?. Plant Mol Biol 2002; b 49:273 - 84; http://dx.doi.org/10.1023/A:1015248926412; PMID: 12036254
- Halliday KJ, Martínez-García JF, Josse E-M. Integration of light and auxin signaling. Cold Spring Harb Perspect Biol 2009; 1:a001586; http://dx.doi.org/10.1101/cshperspect.a001586; PMID: 20457562
- Kim JI, Murphy AS, Baek D, Lee S-W, Yun D-J, Bressan RA, Narasimhan ML. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J Exp Bot 2011; 62:3981 - 92; http://dx.doi.org/10.1093/jxb/err094; PMID: 21511905
- Kim JI, Sharkhuu A, Jin JB, Li P, Jeong JC, Baek D, Lee SY, Blakeslee JJ, Murphy AS, Bohnert HJ, et al. yucca6, a dominant mutation in Arabidopsis, affects auxin accumulation and auxin-related phenotypes. Plant Physiol 2007; 145:722 - 35; http://dx.doi.org/10.1104/pp.107.104935; PMID: 17885085
- Pilet P-E, Phipps J. Inhibition of auxin catabolism in relation to the chlorophyll content of the tissues. Planta 1968; 80:82 - 8; http://dx.doi.org/10.1007/BF00387192
- Cazzonelli CI, Roberts AC, Carmody ME, Pogson BJ. Transcriptional control of SET DOMAIN GROUP 8 and CAROTENOID ISOMERASE during Arabidopsis development. Mol Plant 2010; 3:174 - 91; http://dx.doi.org/10.1093/mp/ssp092; PMID: 19952001
