Abstract
Symplasmic communication via plasmodesmata (PD) is part of the system of information exchange between plant cells. Molecules that pass through the PD include ions, some hormones, minerals, amino acids, and sugars but also proteins, transcription factors, and different classes of RNA, and as such PD can participate in the coordination of plant growth and development. This review summarizes the current literature on this subject and the role of PD in signal exchange, the importance of symplasmic communication and symplasmic domains in plant cell differentiation, and highlights the future prospective in the exploration of PD functions in plants. Moreover, this review also describes the potential use of barley root epidermis and non-zygotic embryogenesis in study of symplasmic communication during cell differentiation.
The presence of PD, channels within the walls between neighboring cells, can be interpreted as the main reason determined at least 2 continuous systems in plants: apoplast and symplasm.Citation1 The apoplast is an extraprotoplasmic region composed of cell walls with microspaces within the cell wall, some intercellular spaces, and the lumina of dead cells.Citation2 The symplasm is a system consisting of all protoplasts interconnected by PD and bounded by continuous plasma membrane.Citation3 Terminology used for the description of these systems in not consistent in the literature and because of it, the terminology proposed by Romberger et al.Citation1 will be used. Namely, in this review we will use the term “symplasm,” and appropriate derivative terms as “symplasmic” or “symplasmically,” as we agree with Romberger et al.Citation1 that the term “symplastic” has another meaning referring to growth of cells within the plant tissue.Citation4 From functional point of view, PD are the main part of the symplast, but we would like to point to another possible spatial system which can be distinguished because of PD structure. Namely, it is worth introducing into the nomenclature the term “endoplasmic symreticulum” as suggested previously,Citation5 that would comprise a third continuous spatial system within a plant body. This system is composed of the endoplasmic reticulum traversing through PD thus creating the unity of the endoplasmic reticulum within the plant body.
Plasmodesmata structure and permeability—present knowledge
PD are structures present in plants,Citation6 algae,Citation7,Citation8 and fungi,Citation9 which comprise cytoplasmic channels that connect the neighboring cells, and allow the communication between individual cells (cell-to-cell communication).Citation10-Citation14 The first report describing these “channels” across neighboring plant cell walls, deduced from observation with the use of conventional microscopy, comes from 1880,Citation6 and name “plasmodesmen” was used for the first time in 1901 by Strasburger.Citation15 During the last few years detailed ultrastructure of PD was described in review papers,Citation16,Citation17 so here we only mention that cytoplasmic channels are surrounded by plasma membrane (PM) internally lined with an appressed ER membrane, termed the desmotubule ().Citation18,Citation19
Figure 1. Schematic representation of the molecular structure of PD and movement of molecules. Description is given in the text.
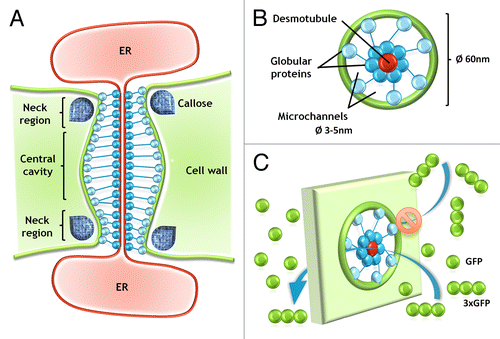
The diameter of PD is not constant and may be reduced until the complete closure inter alia by the deposition of callose (β-1,3-glucan) in the neck regions of PD.Citation20,Citation21 Deposition of this polysaccharide depends on the activity of 2 enzyme groups: β-1,3-glucan synthase that produces callose, and β-1,3-glucanase responsible for callose degradation.Citation22,Citation23 The diameter and permeability of PD may be modified during cell development or in response to the external conditions, like temperature, pathogen attack, or wounding.Citation14,Citation21 The permeability of PD is also limited, by the diameter of microchannels (), and the value of SEL (size exclusion limit), described in units of mass, is used in most cases to determine which molecules can pass through the PD, what is an indicator of the maximal molecular size of the molecule/molecules traversed through PD.Citation24 Many studies on the communication via PD are based on the transport of low molecular fluorochromes, fluorescent labeled dextrans, or green fluorescent protein (GFP), which allows to compare PD permeability for molecules of different sizes.Citation25-Citation29 Sometimes to determine the maximum size of molecule, that may migrate through PD, GFP molecules, and complexes of 2 or 3 GFPs molecules (2xGFP/3xGFP) are being used.Citation30,Citation31 It is important to take into consideration that in such cases the SEL can be between 27–81 kDa. However, it must be understood not as a diameter of microchannels participating in GFP movement, but the parameter describing the molecule size, including its length, which can influence the movement of the molecules in question. The correlation between increasing size of GFP complexes and the reduced permeability of PD is obvious,Citation30 but it cannot be excluded that 3, connected in series, molecules of GFP, and one single GFP may move through PD with the same diameter of microchannels (). Moreover molecules with a lower molecular weight may have a larger diameter than the molecules of larger weight ().Citation32,Citation33 This explains why the description of PD microchannel diameter using of the radius of molecules – MEL (molecular exclusion limit) is more accurate than molecule weight.Citation34-Citation38
Table 1. Comparison of the molecular weight and diameter of some of the molecules used in the analysis of symplasmic communication.
Symplasmic transport—route for molecules including macromolecules
Initially it was postulated that PD are an intracellular channels for the diffusion of small molecules, such as ions or sugars.Citation6,Citation39 However, subsequent studies on the PD described these structures as dynamic gateways actively transporting or blocking transport of macromolecules: proteins and RNAs.Citation37,Citation40,Citation41 The first information regarding macromolecules transported through PD was based on the studies on movement protein (MP) encoded by Tobacco mosaic virus.Citation24,Citation42 MPs interact with the PD and may increase their diameter, break symplasmic isolation of cells, and spread viruses through the plant tissues.Citation43,Citation44 Further it was discovered that plant proteins may pass via PD, using a similar mechanism as MPs, and all proteins transported symplasmically between cells have been called as non-cell-autonomous proteins (NCAPs).Citation45 To NCAPs are included for example: transcription factors, like KNOTTED1 (KN1), which plays a crucial role in maize meristem organization,Citation46 or cell-fate-deciding proteins, involved for example in the root hair development, like TRANSPARENT TESTA GLABRA1 (TTG1),Citation47 CAPRICE (CPC),Citation48 or CAPRICE-LIKE MYB3 (CPL3).Citation49 The movement of NCAPs related to cell differentiation is regulated by as yet unknown mechanisms, that precisely guide proteins to the specific individual cells (like TTG1, CPC, CPL) or cell layers (like KN1; for a review see refs. Citation16,Citation17). Interactions between NCAPs and PD are not well understood, although the motifs crucial for symplasmic transport of different proteins have been described.Citation17,Citation50,Citation51
A second group of molecules that may be symplasmically transported between neighboring cells are RNAs including small RNA (sRNA) involved in RNA silencing.Citation52 sRNAs are classified as short (21–24 nucleotides), non-coding molecules that have an influence on transcriptional and post-transcriptional regulation of the target sequences.Citation53 Transport of sRNA between plant cells and their role in the cell differentiation or in the response to the viruses infection is well understood (for a review see refs. Citation54,Citation55). One of the best documented examples is the description of 2 microRNA molecules: miR165a and miR166b, involved in the development of vascular tissue in Arabidopsis thaliana root.Citation56,Citation57 Both miRNAs expressed in root endodermis, non-cell-autonomously suppress the expression of PHABULOSA (PHB), class III HD-ZIP transcription factor. And this suppression of PHB in the peripheral root stele is required for the xylem differentiation.Citation56 Also the gradual distribution of PHB among the root stele, due to the miR165a/ miR166b silencing, is crucial for the differentiation of pericycle and ground tissue pattering in Arabidopsis roots.Citation57 Moreover, the expression of MIR165a/MIR166b is activated in the endodermis by SHORT-ROOT (SHR) transcription factor, that is also transported via PD,Citation56,Citation58 these data indicate that NCAPs play a role in cell differentiation at multiple levels and may interact with others NCAPs or key cell-fate deciding proteins.
Symplasmic communication/isolation—basic definition
The discovery that the plant body is divided into regions consisting of cells which are not connected by PD, or in which such connections are temporally closed or diminished, resulted in the terms “symplasmic domains” and “subdomains” or “symplasmic fields” being used.Citation59 A symplasmic domain is a cell or group of cells which are connected by PD between each other, but on the border of a domain is not connected by functional PD with the neighbor cells or connection is diminished. If such a lack of connection by PD is permanent the domain is called “permanent symplasmic domain” and the best example is stomata cells.Citation60 Much more interesting are the “temporary symplasmic domains,” which consist of cells, or group of cells which only temporally closed PD on the domain border or the movement of molecules through PD is diminished quantitative or qualitative.Citation61 Sometimes within the domain, subdomains can be distinguished.Citation62 Temporal domains are more interesting because the analysis of their appearance/disappearance and the mechanisms involved in their function can provide answers to the role of symplasmic domains in plant growth and development and the spatio-temporal correlation between symplasmic domains and cell differentiation.Citation59
It must be noticed that apart from “symplasmic domain,” the term “symplasmic field” exists. This term was introduced for the description of symplasmic isolated areas present in the apical meristem.Citation63,Citation64 The authors proposed such a nomenclature because apical meristem is different from mature tissues (for which term symplasmic domain was introduced)Citation65 in the way that apical meristem is continuously renewing its cellular composition.Citation64
Correlation between symplasmic communication/isolation and cell differentiation
It is worthy of note that changes in gene expression are correlated with the changes in symplasmic communication based on the number of PD, or their permeability as demonstrated during crown gall development, where no PD exists on the border between the tumor and the rest of the plant,Citation66 in Chara vulgaris closing of PD is the necessary for proper spermatogenesisCitation67 or in the case of Onoclea sensibility where disturbing of the connection by PD resulted in possessing of the cells totipotency.Citation68
As noted previously, the role of PD in cell differentiation is based on the discovery that plant cells can communicate by PD with neighboring cells within the symplasmic domain and share with each other common components and behavior.Citation69 It is known that cell differentiation is correlated with the formation of symplasmic domains or subdomains (for a review see refs. Citation17,Citation70,Citation71). It is also known that cells in symplasmic connection (within symplasmic domain) are characterized by the same direction and frequency of cell division,Citation72 that the more advanced the state of cell differentiation the lower symplasmic connection,Citation30,Citation62,Citation73 and disturbance in symplasmic transport causes changes in normal plant growth and development.Citation74,Citation75 It is postulated, that changes in the direction of cell differentiation are correlated with the changes in symplasmic communication. For example, in callus of Cocos nucifera embryogenic cells were separated symplasmically from the explant at the globular stage of somatic embryo development suggesting that the acquisition of embryogenic competence is preceded by closing of PD.Citation76 Another example from studies on somatic embryogenesis of symplasmic isolation as a main factor allowing changes in cell fate comes from studies on Quercus suber L. callus where dividing cells (future somatic embryos) were symplasmically isolated from the explant.Citation77
Zygotic and non-zygotic embryogenesis—a good model for symplasmic communication studies
The hypothesis about the role of the symplasmic isolation of cells during zygotic embryogenesis was first postulated after an analysis of the ultrastructure of developing embryos in different plant species. It was shown that during the early stages of zygotic embryogenesis, the embryo sac-forming meiocyte is not connected to adjacent cells by PD.Citation78 Moreover, for the embryo sac of Capsella bursa-pastoris it was shown that the egg is connected by PD to the synergids at first but that these connections disappear after fertilisation, and from that moment the young zygote is not connected to other cells by PD and additionally that the sac is isolated from the nucellar cells by the absence of PD.Citation78 A detailed analysis of the symplasmic communication using fluorescent tracers before and after fertilisation of a Torenia fourieri embryo sac confirmed that the functional connection between the central cell and the egg apparatus cells by PD decreased after fertilisation.Citation79 These results point not only to the problem of cell-to-cell communication during morphogenesis, but also to the important role of the symplasmic isolation of different genotypes and generations.Citation80
The best-described correlation between symplasmic communication and development is the zygotic embryogenesis of Arabidopsis.Citation74 These studies showed that the zygotic embryo is a single symplasmic domain during all of the stages of development only in the case of low molecular weight molecules (0.5 kDa). However, their movement through PD decreased with the more advanced developmental stages in the case of larger molecules (10 kDa F-dextran).Citation74 The use of transgenic lines with GFP allowed the symplasmic subdomains that are correlated with the embryo tissues and organ development to be determined.Citation25,Citation30,Citation74,Citation81 It is important to note that other phenomena such as the unidirectional movement through PD during zygotic embryogenesis have also been described. It has been shown that substances move between the suspensor and embryo in both directions in the case of globular stage zygotic embryos. When embryos shift in symmetry from radial to bilateral symmetry, communication by PD was only from the embryo to the suspensor.Citation30,Citation81 Detailed information about symplasmic isolation/communication during the zygotic embryogenesis has been well described in the literature recently.Citation82
During zygotic embryogenesis, it is difficult to study the mechanism underlying the regulation of this developmental process for many reasons.Citation83,Citation84 That is why non-zygotic embryogenesis (including somatic embryogenesis and androgenesis) has become a very good experimental system for studies on the mechanisms that control embryogenesis and embryo development at different organization levels including symplasmic communication between the explant and embryogenic cells and within non-zygotic embryos during histo- and organogenesis.
Somatic embryogenesis can be divided into at least 2 phases: 1) changes that take place within the explant that lead to the resumption of totipotency, thus allowing somatic embryo development, and 2) within the somatic embryo during the development of the embryo tissues and organs. In the case of explant cells, the main question is what influences the cells within the explant to develop in different directions and why will only some of them develop into somatic embryos? Many factors/reasons have been postulated and among them symplasmic communication seems to play an important role in the regulation of explant cell differentiation during somatic embryogenesis.Citation85 It has been postulated that such isolation is a prerequisite for changes in the cell developmental program.Citation86 The information that is available in the literature concerns an ultrastructural analysis of the explant with attention being paid to the presence of PD between groups of explant cells with different features of their walls and cytoplasm. However, it must be noted that the presence of PD does not mean that intercellular connections are involved in the exchange of “information” because PD can be not functional, but the lack of PD on the “borders” between different groups of cells within the explant indicates a lack of symplasmic communication.
Some information about the role of cell-to-cell communication by PD within the explant comes from experiments using plasmolysis as a factor that disturbs the cytoplasmic connection by PD. In the case of Panax ginseng,Citation87 pretreatment with sucrose caused plasmolysis, and the number of somatic embryos increased 4-fold in comparison to untreated explants.Citation87,Citation88 Moreover, such results also showed that somatic embryos that developed from a preplasmolysed explant were of a single-cell origin in comparison to untreated explants. Similar results were obtained for Eleutherococcus senticosus, Oncidium Sharry Baby, Panax japonicas, or Daucus carota where plasmolysis of an explant caused an increase in the frequencies of somatic embryos.Citation89-Citation91 Plasmolysis as a factor that breaks the PD connection between explant cells may suggest that each explant cell behaves as a single cell, which allows these cells to develop in different directions and in this case allows the reprogramming of explant cells into embryogenically competent cells and finally induces somatic-embryo development.Citation90 According to these results, changes in the cell-to-cell communication between the explant cells are a prerequisite to changing cell fate.
An analysis of the morphology of Theobroma cacao L. explants showed that the frequency of PD influences somatic embryogenesis.Citation92 Namely, that in the epidermal cells within the staminodes, tissues that are involved in primary somatic embryogenesis, the frequencies of PD were higher in comparison to the tissue explants for secondary embryo production. The authors suggested that it is possible that the higher level of intercellular connections that is seen in staminode tissues is in part responsible for the higher degree of cellular co-ordination that is seen in primary embryogenesis as compared with secondary embryogenesis.Citation92 In the callus of Panax quinquefolium in which the authors distinguished a few kinds of cells such as parenchymatous, initial embryogenic, grouped embryogenic cells, and embryoid cells, only in the last were many PD detected.Citation93
An ultrastructural analysis of embryogenic cell clusters of Cephalotaxus harringtonia showed differences in the outer cell wall thickness of embryogenic masses, and the frequencies of PD, which were abundant in the walls between embryogenic cells.Citation94 Similar results were obtained earlier in callus that was derived from Citrus sinensis, which showed that cells that were destined to form new proembryoids became surrounded by greatly thickened cell walls without PD.Citation95 An analysis of androgenesis in Zea mays and Triticum aestivum also showed that cells that realize the same developmental program are connected by numerous intercellular connections.Citation96,Citation97
To date, the only information about cell-to-cell movement through PD between explant cells and the somatic embryo comes from analyses using low-molecular weight fluorochrome on the example of Arabidopsis somatic embryogenesis, which showed that at least for some time, the somatic embryo is symplasmically isolated from the explant.Citation85 These results also showed that the physical isolation of cells by the deposition of cutin may be a factor that allows the changes in the direction of cell differentiation within the explant.Citation85 An analysis of the distribution of the low-molecular weight symplasmic tracers (HPTS and uncaged fluorescein) during barley androgenesis indicates the symplasmic isolation of the protodermis from the underlying cells of the late globular embryo stage onwards, and the embryonic organs at the mature stage of development, which allows the conclusion that in androgenic embryos in Hordeum vulgare the existence of symplasmic domains correlates with tissue and organ development.Citation62 Similar studies were performed on the somatic embryos of Arabidopsis.Citation85,Citation98,Citation99 An analysis of symplasmic communication was performed using various fluorescent tracers with different molecular weights and cytoplasm soluble GFP being expressed under the control of an STM promoter.Citation99 An analysis of symplasmic communication showed that at the beginning of the culture, PD connectivity between the cells of explants increased. Further changes in symplasmic communication were observed after the next few days of the culture and were correlated with the cells divisions of explants. The changes manifested themselves as a restriction of symplasmic communication on the borders between the dividing and non-dividing cells, which indicated that the parts of the explants that were engaged in morphological processes were isolated from those that were not involved in cell fate changes. Moreover, symplasmic isolation between the somatic embryos and explants was detected. Isolation of the dividing cells from the rest of the plant body was also observed in the case of 35S::BBM transgenic seedlings. Meristematic cells at the cotyledons were isolated from the remaining cells. Furthermore, small groups of dividing cells, which give rise to somatic embryos, were symplasmically isolated from the neighborhood.Citation99 These results suggest that the symplasmic connection of embryogenic competent cells is restricted at this stage of embryogenesis. The results that were obtained showed a correlation between the decrease in symplasmic communication and changes in cell fate and somatic embryogenesis.
Studies using low-molecular symplasmic transport fluorochromes in both species, Arabidopsis and barley, showed that symplasmic domains correlated with the differentiation in the first place, the protodermis, and afterwards other embryo tissues and organs. In general, these data showed a correlation between the decrease in symplasmic communication and changes in the cell differentiation of non-zygotic embryos.Citation99 The data obtained from these studies of somatic embryogenesis and androgenesis indicate that in this type of embryogenesis, the general rules related to the correlation between symplasmic communication and plant development are the same or similar as in the case of zygotic embryogenesis. These data also suggest that cell-to-cell communication is a universal mechanism that is involved in regulation of cell differentiation.
On the whole, therefore, the critical evidence supports the view that resumption of totipotency that leads to embryogenesis in cultured cells is preceded by cell isolation and that differentiation of somatic embryo cells into tissues and the formation of a somatic embryo organ is correlated with changes in symplasmic communication.
Barley root epidermis: a new model for symplasmic communication studies
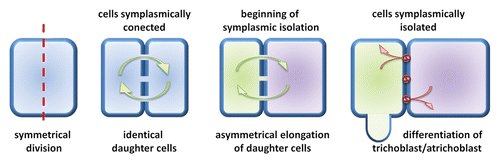
The recent data has suggested that barley root epidermis may be a useful model for investigation of the role of symplasmic communication in plant cell differentiation.Citation73 The root epidermis is composed of 2 types of cells: those producing root hairs (trichoblasts) and non-root hair cells (atrichoblasts).Citation100 In the meristematic zone of root all epidermal cells are similar in: size, shape, composition, and number of organelles, amount of cytoplasm, or number of molecules localized in cytoplasm.Citation101 Later, in the differentiation zone of root, cells start to differentiate into trichoblasts and atrichoblasts,Citation102 and 2 neighboring cells can realize different developmental programmes.Citation103 From the previous studies, it is known that in this situation the neighboring cells are usually symplasmically isolated.Citation74 Similar results were obtained in studies on Arabidopsis root epidermis, where after microinjection into the individual root hair or non-root hair cell, fluorescent tracers (Lucifer Yellow and carboxyfluorescein) were not transported to the surrounding cells.Citation104 It was also shown that, during the differentiation of barley root epidermal cells, symplasmic communication is restricted in wild type plants and in the mature root hair zone epidermal cells are still symplasmically isolated.Citation73 Moreover, for 2 allelic root hairless mutants rhl1.a and rhl1.b isolation of epidermal cells was not present. Immunogold detection of callose with the use of the transmission electron microscope revealed that in PD localized between epidermal cells in the differentiation zone of wild type root higher number of callose molecules were deposited, in comparison to the comparable zone of root in root hairless mutant. In mutant plants only single callose molecules were present, and their number was similar to this in the meristematic zone of root.Citation73 Results obtained for Arabidopsis and barley clearly indicate a strong correlation between differentiation of root epidermal cells and restriction of symplasmic communication.Citation73,Citation104 However, there are a few alternative mechanisms of establishing the root epidermal patterns in plants: symmetrical or asymmetrical division of mother cell, asymmetrical expansion of daughter cells, or some unknown factor that induce root hair development of individual cells.Citation105 In the Arabidopsis root, epidermal cells are arranged in files of trichoblasts and atrichoblasts, and in the early stage of development, in the meristematic region, the fate of cells is determined.Citation101 Whereas in barley the shootward-last division of epidermal cell (division localized the furthest from the root meristem) is symmetric and both daughter cells are identical, but afterwards cells start to expand asymmetrically ().Citation106 The rootward cell (localized closer to the root meristem) is shorter, contains more dense cytoplasm, as well as higher number of mitochondria in comparison to the longer shootward cell. During the differentiation process only shorter cells produce root hairs, what indicates that asymmetric cell expansion plays a key role in root epidermal pattering in barley.Citation106 Additionally, in barley root hairless mutants rhl1.bCitation107 and brb (bald root barley)Citation108 all epidermal cells are similar in size and cytoplasm density, what confirms the hypothesis about importance of asymmetrical expansion in root hair development.Citation106 Current data indicate that the root epidermis of barley may be a new useful model for the studies of symplasmic communication, because in this case, 2 identical daughter cells start asymmetric expansion, which is related to the restriction of symplasmic communication. Disturbances in symplasmic isolation and/or asymmetric cell expansion are responsible for root hairless phenotype – trait that is easily observed during phenotypic analysis.
Future Perspectives
Since plant development primarily depends upon positional information, it would be useful to know the influence of this factor on PD function. Our knowledge regarding the mechanism by which some molecules can pass through the PD and others cannot is still obscure. Currently, information about the proteins connected with the PD structure and function is limited. However, recent data indicate that intercellular connections are not just conduits for macromolecular transport, but also represent sites for glycan synthesis and, potentially, post translational modification.Citation109 Recent studies of grafting have shown that cells exchange chloroplasts by PD between genetically distinct cell types,Citation110,Citation111 which points to an open question regarding the similarity between PD and tunneling nanotubules (TNT) discovered in animal cells in the correlation of growth and development. The future direction of studies must answer the question as to how PD function and formation are integrated with intracellular signaling pathways and plant physiology.Citation16
| Abbreviations: | ||
| CFDA- 5-(and-6) | = | Carboxyfluorescein Diacetate |
| ER | = | endoplasmic reticulum |
| GFP | = | green fluorescent protein |
| HPTS | = | 8-Hydroxypyrene-1,3,6-trisulfonic acid |
| MP | = | movement protein |
| NCAP | = | non-cell-autonomous proteins |
| PD | = | plasmodesmata |
| PM | = | plasma membrane |
| TNT | = | tunneling nanotubules |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by grants from the Polish National Science Center: 2011/01/M/NZ2/02979 and 2013/08/T/NZ3/00811.
References
- Romberger JA, Hejnowicz Z, Hill JF. Plant structure: function and development. Caldwel, NJ USA: The Blackburn Press, 2004
- Sattelmacher B. The apoplast and its significance for plant mineral nutrition. New Phytol 2001; 149:167 - 92; http://dx.doi.org/10.1046/j.1469-8137.2001.00034.x
- Erickson RO. Symplastic growth and symplasmic transport. Plant Physiol 1986; 82:1153 - 1153; http://dx.doi.org/10.1104/pp.82.4.1153; PMID: 16665152
- Priestley JH. Studies in the physiology of cambial activity. II. The concept of sliding growth. New Phytol 1930; 29:96 - 140; http://dx.doi.org/10.1111/j.1469-8137.1930.tb06983.x
- Hejnowicz Z, Krause E, Glebicki K, Sievers A. Propagated fluctuations of the electric potential in the apoplasm of Lepidium sativum L. roots. Planta 1991; 186:127 - 34; http://dx.doi.org/10.1007/BF00201508; PMID: 24186585
- Tangl E.. Ueber offene Communicationen zwischen den Zellen des Endosperms einiger Samen. Jb wiss Bot 1880; 12:170 - 190
- Kwiatkowska M, Maszewski J. Changes in the occurrence and ultrastructure of plasmodesmata in antheridia of Chara vulgaris L. during different stages of spermatogenesis. Protoplasma 1986; 132:179 - 88; http://dx.doi.org/10.1007/BF01276998
- Terauchi M, Nagasato C, Kajimura N, Mineyuki Y, Okuda K, Katsaros C, Motomura T. Ultrastructural study of plasmodesmata in the brown alga Dictyota dichotoma (Dictyotales, Phaeophyceae). Planta 2012; 236:1013 - 26; http://dx.doi.org/10.1007/s00425-012-1656-4; PMID: 22547029
- Hawker LE, Gooday MA, Bracker CE. Plasmodesmata in fungal cell walls. Nature 1996; 212:635; http://dx.doi.org/10.1038/212635a0
- Kurata T, Okada K, Wada T. Intercellular movement of transcription factors. Curr Opin Plant Biol 2005; 8:600 - 5; http://dx.doi.org/10.1016/j.pbi.2005.09.005; PMID: 16182599
- Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol 2008; 6:e7; http://dx.doi.org/10.1371/journal.pbio.0060007; PMID: 18215111
- Faulkner C, Maule A. Opportunities and successes in the search for plasmodesmal proteins. Protoplasma 2011; 248:27 - 38; http://dx.doi.org/10.1007/s00709-010-0213-x; PMID: 20922549
- Ueki S, Citovsky V. To gate, or not to gate: regulatory mechanisms for intercellular protein transport and virus movement in plants. Mol Plant 2011; 4:782 - 93; http://dx.doi.org/10.1093/mp/ssr060; PMID: 21746703
- Burch-Smith TM, Zambryski PC. Plasmodesmata paradigm shift: regulation from without versus within. Annu Rev Plant Biol 2012; 63:239 - 60; http://dx.doi.org/10.1146/annurev-arplant-042811-105453; PMID: 22136566
- Strasburger E.. Über plasmaverbindungen pflanzlicher zellen. Jb wiss Bot 1901; 36:493 - 610
- Brunkard JO, Runkel AM, Zambryski PC. Plasmodesmata dynamics are coordinated by intracellular signaling pathways. Curr Opin Plant Biol 2013; 16:614 - 20; http://dx.doi.org/10.1016/j.pbi.2013.07.007; PMID: 23978390
- Kragler F. Plasmodesmata: intercellular tunnels facilitating transport of macromolecules in plants. Cell Tissue Res 2013; 352:49 - 58; http://dx.doi.org/10.1007/s00441-012-1550-1; PMID: 23370600
- Ding B, Turgeon R, Parthasarathy MV. Substructure of freeze-substituted plasmodesmata. Protoplasma 1992; 169:28 - 41; http://dx.doi.org/10.1007/BF01343367
- Lee JY, Yoo BC, Lucas WJ. Parallels between nuclear-pore and plasmodesmal trafficking of information molecules. Planta 2000; 210:177 - 87; http://dx.doi.org/10.1007/PL00008124; PMID: 10664123
- Northcote DH, Davey R, Lay J. Use of antisera to localize callose, xylan and arabinogalactan in the cell-plate, primary and secondary walls of plant cells. Planta 1989; 178:353 - 66; http://dx.doi.org/10.1007/BF00391863; PMID: 24212902
- Zavaliev R, Ueki S, Epel BL, Citovsky V. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 2011; 248:117 - 30; http://dx.doi.org/10.1007/s00709-010-0247-0; PMID: 21116665
- Levy A, Erlanger M, Rosenthal M, Epel BL. A plasmodesmata-associated β-1,3-glucanase in Arabidopsis. Plant J 2007; 49:669 - 82; http://dx.doi.org/10.1111/j.1365-313X.2006.02986.x; PMID: 17270015
- Chen XY, Kim JY. Callose synthesis in higher plants. Plant Signal Behav 2009; 4:489 - 92; http://dx.doi.org/10.4161/psb.4.6.8359; PMID: 19816126
- Wolf S, Deom CM, Beachy RN, Lucas WJ. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 1989; 246:377 - 9; http://dx.doi.org/10.1126/science.246.4928.377; PMID: 16552920
- Crawford KM, Zambryski PC. Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr Biol 2000; 10:1032 - 40; http://dx.doi.org/10.1016/S0960-9822(00)00657-6; PMID: 10996070
- Xu M, Cho E, Burch-Smith TM, Zambryski PC. Plasmodesmata formation and cell-to-cell transport are reduced in decreased size exclusion limit 1 during embryogenesis in Arabidopsis. Proc Natl Acad Sci U S A 2012; 109:5098 - 103; http://dx.doi.org/10.1073/pnas.1202919109; PMID: 22411811
- Yoo SC, Chen C, Rojas M, Daimon Y, Ham BK, Araki T, Lucas WJ. Phloem long-distance delivery of FLOWERING LOCUS T (FT) to the apex. Plant J 2013; 75:456 - 68; http://dx.doi.org/10.1111/tpj.12213; PMID: 23607279
- Guseman JM, Lee JS, Bogenschutz NL, Peterson KM, Virata RE, Xie B, Kanaoka MM, Hong Z, Torii KU. Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8). Development 2010; 137:1731 - 41; http://dx.doi.org/10.1242/dev.049197; PMID: 20430748
- Wolters H, Anders N, Geldner N, Gavidia R, Jürgens G. Coordination of apical and basal embryo development revealed by tissue-specific GNOM functions. Development 2011; 138:117 - 26; http://dx.doi.org/10.1242/dev.059147; PMID: 21138974
- Kim I, Zambryski PC. Cell-to-cell communication via plasmodesmata during Arabidopsis embryogenesis. Curr Opin Plant Biol 2005; 8:593 - 9; http://dx.doi.org/10.1016/j.pbi.2005.09.013; PMID: 16207533
- Kobayashi K, Zambryski P. RNA silencing and its cell-to-cell spread during Arabidopsis embryogenesis. Plant J 2007; 50:597 - 604; http://dx.doi.org/10.1111/j.1365-313X.2007.03073.x; PMID: 17419839
- Fisher DB, Cash-Clark CE. Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol 2000; 123:125 - 38; http://dx.doi.org/10.1104/pp.123.1.125; PMID: 10806231
- Kurczynska EU. Symplasmic communication: terminology, fluorochromes and Arabidopsis thaliana embryogenesis. Post Biol Kom 2008; 35:31 - 43
- Terry BR, Robards AW. Hydrodynamic radius alone governs the mobility of molecules through plasmodesmata. Planta 1987; 171:145 - 57; http://dx.doi.org/10.1007/BF00391090; PMID: 24227322
- Baron-Epel O, Hernandez D, Jiang L-W, Meiners S, Schindler M. Dynamic continuity of cytoplasmic and membrane compartments between plant cells. J Cell Biol 1988; 106:715 - 21; http://dx.doi.org/10.1083/jcb.106.3.715; PMID: 3346323
- Kempers R, van Bel AJ. Symplasmic connections between sieve element and companion cell in the stem phloem of Vicia faba L. have a molecular exclusion limit of at least 10 kDa. Planta 1997; 201:195 - 201; http://dx.doi.org/10.1007/BF01007704
- Roberts AG, Oparka KJ. Plasmodesmata and the control of symplastic transport. Plant Cell Environ 2003; 26:103 - 24; http://dx.doi.org/10.1046/j.1365-3040.2003.00950.x
- Marzec M, Kurczynska EU. Symplasmic communication/isolation and plant cell differentiation. Post Biol Kom 2008; 35:369 - 89
- Jones MG, Dropkin VH. Scanning electron microscopy in nematode-induced giant transfer cells. Cytobios 1976; 15:149 - 61; PMID: 1001022
- Robards AW, Lucas WJ. Plasmodesmata. Annu Rev Plant Physiol Plant Mol Biol 1990; 41:369 - 419; http://dx.doi.org/10.1146/annurev.pp.41.060190.002101
- Zambryski P, Crawford K. Plasmodesmata: gatekeepers for cell-to-cell transport of developmental signals in plants. Annu Rev Cell Dev Biol 2000; 16:393 - 421; http://dx.doi.org/10.1146/annurev.cellbio.16.1.393; PMID: 11031242
- Wolf S, Deom CM, Beachy R, Lucas WJ. Plasmodesmatal function is probed using transgenic tobacco plants that express a virus movement protein. Plant Cell 1991; 3:593 - 604; PMID: 1726784
- Wolf S, Lucas WJ. Virus movement proteins and other molecular probes of plasmodesmal function. Plant Cell Environ 1994; 17:573 - 85; http://dx.doi.org/10.1111/j.1365-3040.1994.tb00150.x
- Waigmann E, Zambryski P. Tobacco mosaic virus movement protein-mediated protein transport between trichome cells. Plant Cell 1995; 7:2069 - 79; PMID: 8718620
- Haywood V, Kragler F, Lucas WJ. Plasmodesmata: pathways for protein and ribonucleoprotein signaling. Plant Cell 2002; 14:Suppl S303 - 25; PMID: 12045285
- Jackson D, Veit B, Hake S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 1994; 120:405 - 13
- Bouyer D, Geier F, Kragler F, Schnittger A, Pesch M, Wester K, Balkunde R, Timmer J, Fleck C, Hülskamp M. Two-dimensional patterning by a trapping/depletion mechanism: the role of TTG1 and GL3 in Arabidopsis trichome formation. PLoS Biol 2008; 6:e141; http://dx.doi.org/10.1371/journal.pbio.0060141; PMID: 18547143
- Kurata T, Ishida T, Kawabata-Awai C, Noguchi M, Hattori S, Sano R, Nagasaka R, Tominaga R, Koshino-Kimura Y, Kato T, et al. Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 2005; 132:5387 - 98; http://dx.doi.org/10.1242/dev.02139; PMID: 16291794
- Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T. Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development 2008; 135:1335 - 45; http://dx.doi.org/10.1242/dev.017947; PMID: 18305006
- Kim JY, Rim Y, Wang J, Jackson D. A novel cell-to-cell trafficking assay indicates that the KNOX homeodomain is necessary and sufficient for intercellular protein and mRNA trafficking. Genes Dev 2005; 19:788 - 93; http://dx.doi.org/10.1101/gad.332805; PMID: 15805469
- Gallagher KL, Benfey PN. Both the conserved GRAS domain and nuclear localization are required for SHORT-ROOT movement. Plant J 2009; 57:785 - 97; http://dx.doi.org/10.1111/j.1365-313X.2008.03735.x; PMID: 19000160
- Hyun TK, Uddin MN, Rim Y, Kim JY. Cell-to-cell trafficking of RNA and RNA silencing through plasmodesmata. Protoplasma 2011; 248:101 - 16; http://dx.doi.org/10.1007/s00709-010-0225-6; PMID: 21042816
- Baulcombe D. RNA silencing in plants. Nature 2004; 431:356 - 63; http://dx.doi.org/10.1038/nature02874; PMID: 15372043
- Molnar A, Melnyk C, Baulcombe DC. Silencing signals in plants: a long journey for small RNAs. Genome Biol 2011; 12:215; http://dx.doi.org/10.1186/gb-2010-11-12-219; PMID: 21235831
- Furuta K, Lichtenberger R, Helariutta Y. The role of mobile small RNA species during root growth and development. Curr Opin Cell Biol 2012; 24:211 - 6; http://dx.doi.org/10.1016/j.ceb.2011.12.005; PMID: 22227227
- Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vatén A, Thitamadee S, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 2010; 465:316 - 21; http://dx.doi.org/10.1038/nature08977; PMID: 20410882
- Miyashima S, Koi S, Hashimoto T, Nakajima K. Non-cell-autonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development 2011; 138:2303 - 13; http://dx.doi.org/10.1242/dev.060491; PMID: 21558378
- Wu S, Gallagher KL. Mobile protein signals in plant development. Curr Opin Plant Biol 2011; 14:563 - 70; http://dx.doi.org/10.1016/j.pbi.2011.06.006; PMID: 21763178
- Lucas WJ, Ham BK, Kim JY. Plasmodesmata - bridging the gap between neighboring plant cells. Trends Cell Biol 2009; 19:495 - 503; http://dx.doi.org/10.1016/j.tcb.2009.07.003; PMID: 19748270
- Erwee MG, Goodwin PB, Bel A. Cell‐cell communication in the leaves of Commelina cyanea and other plants. Plant Cell Environ 1985; 8:173 - 8
- Ehlers K, Binding H, Kollmann R. The formation of symplasmic domains by plugging of plasmodesmata: a general event in plant morphogenesis?. Protoplasma 1999; 209:181 - 92; http://dx.doi.org/10.1007/BF01453447
- Wrobel J, Barlow PW, Gorka K, Nabialkowska D, Kurczynska EU. Histology and symplasmic tracer distribution during development of barley androgenic embryos. Planta 2011; 233:873 - 81; http://dx.doi.org/10.1007/s00425-010-1345-0; PMID: 21225281
- Rinne PL, van der Schoot C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 1998; 125:1477 - 85; PMID: 9502728
- Rinne P, Rinne PL, van der Schoot C. Networks for shoot design. Trends Plant Sci 1999; 4:31 - 7; http://dx.doi.org/10.1016/S1360-1385(98)01362-4; PMID: 10234268
- Erwee MG, Goodwin PB. Characterisation of the Egeria densa Planch. leaf symplast : Inhibition of the intercellular movement of fluorescent probes by group II ions. Planta 1983; 158:320 - 8; http://dx.doi.org/10.1007/BF00397334; PMID: 24264752
- Stahl Y, Simon R. Gated communities: apoplastic and symplastic signals converge at plasmodesmata to control cell fates. J Exp Bot 2013; 64:5237 - 41; http://dx.doi.org/10.1093/jxb/ert245; PMID: 23975796
- Kwiatkowska M, Maszewski J. Plasmodesmata between synchronously and asynchronously developing cells of the antheridial filaments of Chara vulgaris L. Protoplasma 1976; 87:317 - 27; http://dx.doi.org/10.1007/BF01624003
- Tucker EB. Analytical studies of dye-coupling between plant cells. In: Parallels in Cell to Cell Junctions in Plants and Animals. Berlin, Heidelberg: Springer: 1990, 239-248.
- Zambryski P. Cell-to-cell transport of proteins and fluorescent tracers via plasmodesmata during plant development. J Cell Biol 2004; 164:165 - 8; http://dx.doi.org/10.1083/jcb.200310048; PMID: 14734529
- Maule AJ, Benitez-Alfonso Y, Faulkner C. Plasmodesmata - membrane tunnels with attitude. Curr Opin Plant Biol 2011; 14:683 - 90; http://dx.doi.org/10.1016/j.pbi.2011.07.007; PMID: 21820942
- Bloemendal S, Kück U. Cell-to-cell communication in plants, animals, and fungi: a comparative review. Naturwissenschaften 2013; 100:3 - 19; http://dx.doi.org/10.1007/s00114-012-0988-z; PMID: 23128987
- Ehlers K, Kollmann R. Primary and secondary plasmodesmata: structure, origin, and functioning. Protoplasma 2001; 216:1 - 30; http://dx.doi.org/10.1007/BF02680127; PMID: 11732191
- Marzec M, Muszynska A, Melzer M, Sas-Nowosielska H, Kurczynska EU. Increased symplasmic permeability in barley root epidermal cells correlates with defects in root hair development. Plant Biol (Stuttg) 2013; •••; http://dx.doi.org/10.1111/plb.12066; PMID: 23927737
- Kim I, Hempel FD, Sha K, Pfluger J, Zambryski PC. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana.. Development 2002; 129:1261 - 72; PMID: 11874921
- Kobayashi K, Otegui MS, Krishnakumar S, Mindrinos M, Zambryski P. INCREASED SIZE EXCLUSION LIMIT 2 encodes a putative DEVH box RNA helicase involved in plasmodesmata function during Arabidopsis embryogenesis. Plant Cell 2007; 19:1885 - 97; http://dx.doi.org/10.1105/tpc.106.045666; PMID: 17601829
- Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Ferriere N, Nicole M. Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot (Lond) 2001; 88:9 - 18; http://dx.doi.org/10.1006/anbo.2001.1408
- Puigderrajols P, Mir G, Molinas M. Ultrastructure of early secondary embryogenesis by multicellular and unicellular pathways in cork oak (Quercus suber L.). Ann Bot (Lond) 2001; 87:179 - 89; http://dx.doi.org/10.1006/anbo.2000.1317
- Schulz SR, Jensen WA. Capsella embryogenesis: the egg, zygote and young embryo. Am J Bot 1968; 55:807 - 19; http://dx.doi.org/10.2307/2440969
- Han Y-Z, Huang B-Q, Zee S-Y, Yuan M. Symplastic communication between the central cell and the egg apparatus cells in the embryo sac of Torenia fournieri Lind. before and during fertilization. Planta 2000; 211:158 - 62; http://dx.doi.org/10.1007/s004250000289; PMID: 10923717
- Ehlers K, van Bel AJE. The physiological and developmental consequence of plasmodesmatal connectivity. In: 11 van Bel, AJE, van Kesteren WJP. Plasmodesmata. Berlin, Heidelberg: Springer-Verlag, 1999; 244-260.
- Stadler R, Lauterbach C, Sauer N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol 2005; 139:701 - 12; http://dx.doi.org/10.1104/pp.105.065607; PMID: 16169962
- Burch-Smith TM, Stonebloom S, Xu M, Zambryski PC. Plasmodesmata during development: re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 2011; 248:61 - 74; http://dx.doi.org/10.1007/s00709-010-0252-3; PMID: 21174132
- Zimmerman JL. Somatic embryogenesis: a model for early development in higher plants. Plant Cell 1993; 5:1411 - 23; PMID: 12271037
- Rao KS. Embryogenesis in flowering plants: Recent approaches and prospects. J Biosci 1996; 21:827 - 41; http://dx.doi.org/10.1007/BF02704724
- Kurczyńska EU, Gaj MD, Ujczak A, Mazur E. Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta 2007; 226:619 - 28; http://dx.doi.org/10.1007/s00425-007-0510-6; PMID: 17406890
- Feher A, Pasternak TP, Dudits D. Transition of somatic plant cells to en embryogenic state. Plant Cell Tissue Organ Cult 2003; 74:201 - 28; http://dx.doi.org/10.1023/A:1024033216561
- Choi YE, Yang DC, Yoon ES, Choi KT. High-efficiency plant production via direct somatic single embryogenesis from preplasmolysed cotyledons of Panax ginseng and possible dormancy of somatic embryos. Plant Cell Rep 1999; 18:493 - 9; http://dx.doi.org/10.1007/s002990050610
- Choi YE, Soh WY. Enhanced somatic single embryo formation by plasmolyzing pretreatment from cultured ginseng cotyledons. Plant Sci 1997; 130:197 - 206; http://dx.doi.org/10.1016/S0168-9452(97)00217-3
- Wetherell DF. Enhanced adventive embryogenesis resulting from plasmolysis of cultured wild carrot cells. Plant Cell Tissue Organ Cult 1984; 3:221 - 7; http://dx.doi.org/10.1007/BF00040341
- Ling You X, Seon Yi J, Eui Choi Y. Cellular change and callose accumulation in zygotic embryos of Eleutherococcus senticosus caused by plasmolyzing pretreatment result in high frequency of single-cell-derived somatic embryogenesis. Protoplasma 2006; 227:105 - 12; http://dx.doi.org/10.1007/s00709-006-0149-3; PMID: 16736252
- You XL, Han JY, Choi YE. Plant regeneration via direct somatic embryogenesis in Panax japonicas.. Plant Biotechnol Rep 2007; 1:5 - 9; http://dx.doi.org/10.1007/s11816-007-0009-4
- Guilitinan M, Maximova S. Recent advances in the tissue culture of cocoa from somatic embryos to bentwood gardens - a short review. In: Proceedings of the International Workshop of the New Technologies and Cocoa Breeding, Kota Kinabalu, Malysia. INGENIC, Reading, UK, 2001; 157-162.
- Li Z, Guo Z, Yingqian ZQ. Ultrastructural characteristics of the embryogenic callus cells of American ginseng. In: Biotechnology in Agriculture. You C, Chen Z, Ding Y, eds. 1993; 317-320.
- Rohr R, Piola F, Pasquier P. Somatic embryogenesis in Cephalotaxus harringtonia Embryo-Megagametophyte co-culture. J For Res 1997; 2:69 - 73; http://dx.doi.org/10.1007/BF02348471
- Button J, Kochba J, Bornman CH. Fine Structure of and embryoid development from embryogenic ovular callus of “Shamouti” orange (Citrus sinensis Osb.). J Exp Bot 1974; 25:446 - 57; http://dx.doi.org/10.1093/jxb/25.2.446
- Bonet FJ, Olmedilla A. Structural changes during early embryogenesis in wheat pollen. Protoplasma 2000; 211:94 - 102; http://dx.doi.org/10.1007/BF01279902
- Testillano PS, Ramírez C, Domenech J, Coronado M-J, Vergne P, Matthys-Rochon E, Risueño MC. Young microspore-derived maize embryos show two domains with defined features also present in zygotic embryogenesis. Int J Dev Biol 2002; 46:1035 - 47; PMID: 12533027
- Kurczyńska EU, Wróbel J, Ujczak A, Gaj MD. Symplasmic communication during somatic embryogenesis of Arabidopsis thaliana L. Heynh. Biol Lett 2005; 42:134 - 5
- Kulinska-Lukaszek K, Kurczynska EU. Symplasmic communication and cell fate changes in Arabidopsis thaliana explants and seedlings in in vitro conditions. BioTechnologia 2012; 93:169
- Cormack RGH. Investigations on the development of root hairs. New Phytol 1935; 34:30 - 54; http://dx.doi.org/10.1111/j.1469-8137.1935.tb06826.x
- Dolan L. Pattern in the root epidermis: an interplay of diffusible signals and cellular geometry. Ann Bot (Lond) 1996; 77:547 - 53; http://dx.doi.org/10.1093/aob/77.6.547
- Kim CM, Dolan L. Root hair development involves asymmetric cell division in Brachypodium distachyon and symmetric division in Oryza sativa.. New Phytol 2011; 192:601 - 10; http://dx.doi.org/10.1111/j.1469-8137.2011.03839.x; PMID: 21848982
- Datta S, Kim CM, Pernas M, Pires ND, Proust H, Tam T, Vijayakumar P, Dolan L. Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil 2011; 346:1 - 14; http://dx.doi.org/10.1007/s11104-011-0845-4
- Duckett CM, Oparka KJ, Prior DA, Dolan L, Roberts K. Dye-coupling in the root epidermis of Arabidopsis is progressively reduced during development. Development 1994; 120:3247 - 55
- Marzec M, Melzer M, Szarejko I. The evolutionary context of root epidermis cell patterning in grasses (Poaceae). Plant Signal Behav 2014; •••:2014
- Marzec M, Melzer M, Szarejko I. Asymmetric growth of root epidermal cells is related to the differentiation of root hair cells in Hordeum vulgare (L.). J Exp Bot 2013; 64:5145 - 55; http://dx.doi.org/10.1093/jxb/ert300; PMID: 24043851
- Szarejko I, Janiak A, Chmielewska B, Nawrot M. Genetic analysis of several root hair mutants of barley. Barley Genet Newsl 2005; 35:36 - 8
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant Soil 2001; 235:211 - 9; http://dx.doi.org/10.1023/A:1011993322286
- Zalepa-King L, Citovsky V. A plasmodesmal glycosyltransferase-like protein. PLoS One 2013; 8:e58025; http://dx.doi.org/10.1371/journal.pone.0058025; PMID: 23469135
- Stegemann S, Bock R. Exchange of genetic material between cells in plant tissue grafts. Science 2009; 324:649 - 51; http://dx.doi.org/10.1126/science.1170397; PMID: 19407205
- Stegemann S, Keuthe M, Greiner S, Bock R. Horizontal transfer of chloroplast genomes between plant species. Proc Natl Acad Sci U S A 2012; 109:2434 - 8; http://dx.doi.org/10.1073/pnas.1114076109; PMID: 22308367