Abstract
In the last century, the mechanism for establishing the root epidermal pattern in grasses was proposed as a differentiating trait that can be used in taxonomic studies and as a useful tool to indicate the relationships between genera. However, knowledge about root hair differentiation in monocots is still scarce. During the last few years, this process has been studied intensively, mainly based on genetics and histological studies. A histological analysis of the root epidermis pattern composed from root hairs (trichoblasts) and non-root hair cells (atrichoblasts), as well as observations of the mechanism of the establishment of this pattern allowed 2 different methods of epidermal cell specialization in monocots to be precisely described. Additionally, a recently published paper describing root hair development in barley shed new light on the evolutionary context of the mechanism of root epidermis cell specialization, which is discussed in the presented work.
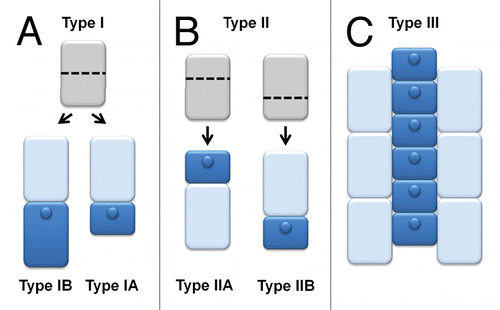
Some root epidermal cells of plants produce root hairs, the tubular outgrowths that increase the surface of the roots.Citation1 The epidermal cells of roots that produce root hairs are called trichoblasts, whereas epidermal cells without the capability of root hair formation are called as atrichoblasts. Three different types of trichoblast/atrichoblast patterns have been described in plants: 1) any cell of the root epidermis can form a root hair (), 2) the epidermis is composed of 2 types of cells of different lengths and only the shorter cells can produce root hairs (), and 3) a striped pattern, when files of root hairs and non-root hair cells are present ().Citation2 The first pattern of the root epidermis is present in many plant species, including dicots, monocots and ferns.Citation1,Citation3 The second pattern of the root epidermis can mainly be found in groups of the oldest land plants, such as Lycopsida or Sphenopsida; in many species of monocots, e.g., in Pooideae and in individual families of dicotyledonous plants, such as Nymphaeceae.Citation2,Citation4 The third pattern of root epidermal cells with files of trichoblasts and atrichoblasts was found in Brassicaceae and their sister families, such as Capparaceae, Tovariceae, and Resedaceae.Citation3,Citation5
Figure 1. Root epidermis patterning in plants. (A) Type 1 in which any root epidermal cell may produce a root hair, (B) Type II in which only the shorter cells produce root hairs, (C) Type III with a striped pattern in which files of root hairs and non-root hair cells are present. (D-E) Two possibilities for the presence of the alternative pattern of shorter and longer cells—(D) the asymmetric shootward-last division or (E) the asymmetric expansion of identical daughter cells after the symmetrical division.
The differentiation of epidermal cells into trichoblasts and atrichoblasts can take place in the meristematic zone of a root during cell divisions or in the elongation zone of a root during cell growth.Citation6 Morphological differences between trichoblasts and atrichoblasts have not been observed in the first pattern of the root epidermis with the exception of the presence of root hair tubes,Citation5 whereas in both the second and the third epidermal cell pattern, the cells that produce root hairs are shorter, have a denser cytoplasm and a reduced vacuolation in comparison to the non-root hair cells.Citation7,Citation8 These differences allow trichoblasts to be distinguished from atrichoblasts, even in the meristematic zone.Citation2
There are 2 main ways that unequal root epidermal cells originate – an asymmetric division of a mother cell or an asymmetric expansion of daughter cells after a symmetric division.Citation9,Citation10 Asymmetric divisions play a crucial role in plant cell differentiation and create cellular diversity—2 unequal daughter cells may start to realize different developing programs directly after the division.Citation11 This happens because the daughter cells differ not only in size but also in their shape, localization, composition, and the number of organelles, the amount of cytoplasm and molecules that are localized in the cytoplasm, such as proteins or RNAs.Citation12 These types of cell divisions lead to the creation of new tissues/organs and are required for many developmental processes, among them plant embryogenesis,Citation13 stomata development,Citation14 and root epidermis patterning.Citation9 In the case of the root epidermis, after the asymmetric division, the shorter daughter cell will develop into a trichoblast, whereas the longer cell will develop into a non-root hair cell (). The shorter cell may be localized closer to the root meristem (called a rootward cell) or closer to the shoot meristem (called a shootward cell).Citation9
However, it is also possible that the shootward-last division of a root epidermal cell is symmetric, but that the 2 identical daughter cells start to expand asymmetrically ().Citation10,Citation15 After a period of asymmetric growth, the daughter cells differ in size, cytoplasm density, the level of vacuolation, and the amount of mitochondria. Only the shorter epidermal cells can produce root hairs, whereas the longer cells become atrichoblasts.
The vast majority of our knowledge about root hair development comes from studies on dicotyledonous species, especially Arabidopsis thaliana, whereas information about monocots is scarce. During the last few years, new genes that are involved in the root hair development in Oryza sativaCitation16,Citation17 and Zea maysCitation18 have been identified and detailed histological studies on the root epidermis of Brachypodium distachyon, rice,Citation10 and Hordeum vulgareCitation11,Citation19 have been performed. Moreover, some comparative analyses of the root epidermal pattern in grasses (Poaceae) were performed in the last century and the application of this kind of analysis for taxonomic studies was proposed.Citation20-Citation22 The first report, which was published in 1939, described 2 types of the shootward-last divisions in Poaceae—type A, when the last division produces unequal daughter cells and type B, when daughter cells after the last division are identical.Citation20 Type A was observed in Phleum pratense L. and Poa trivialis L. (currently classified in the Pooideae subfamily)Citation23; type B when the daughter cells were indistinguishable in size and content was described in Sporobolus cryptandrus (Torr.) Gray (currently classified in the Chloridoideae subfamily) (, ).Citation23 The root epidermal cells of Chloris gayana Knuth. (currently classified in the Chloridoideae subfamily)Citation23 was described as an intermediate type between types A and B () because the last division appeared to be slightly unequal.Citation20 In subsequent reports, 6 species (Phalaris arundinacea L., Poa pratensis L, Phleum pretense L., Agrostis alba L., Agrostis tenuis Sibth., and Beckmannia syzigachne Steud.) from the Pooideae subfamily (according to the current classification)Citation23 were classified as type A,Citation21,Citation24 whereas representatives of the current Panicoideae subfamily (Panicum capillare L., Digitaria sanguinalis L., Miscanthus sinensis, Andropogon scoparius Michx.) showed the B type of cell epidermis divisions (; ).Citation21
Figure 2. Phylogeny of Poaceae from the Catalogue of New World Grasses. Blue indicates subfamilies in which symmetric shootward-last divisions was present, red indicates asymmetric shootward-last divisions, subfamilies that were not investigated are marked with white, the number of analyzed species can be find in brackets.
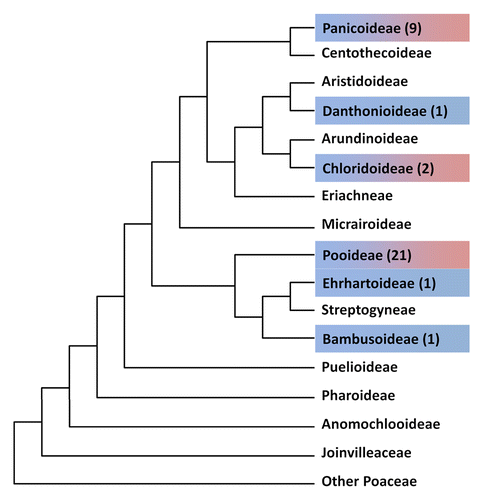
Table 1. Species of the Poaceae family with a known root epidermis pattern
According to the taxonomy of the previous century, any species representing type A of the root epidermis was classified into the Festucoideae subfamily and species expressing type B were classified into the Panicoideae subfamily, thus the type A was renamed as a “festucoid type” and type B as a “panicoid type.”Citation21 All data that was presented indicate that the development of root hairs in Poaceae was considered to be an important taxonomic feature that correlated with other taxonomic characteristics, such as the number and size of chromosomes, embryo structure or leaf anatomy.Citation20,Citation21,Citation24 During the next large-scale analysis of monocot species in which 82 species were studied, one of the main features of root morphology was the presence of alternating long and short cells, but the study did not investigate the shootward-last division.Citation22 We now know that the occurrence of asymmetric cells may be caused by asymmetric divisions or asymmetric expansions of daughter cells after a symmetric division.Citation10,Citation15 Authors described root hair development in some of plants as “intermediate” when classification as a festucoid or panicoid type was impossible.Citation22 This report indicated the importance of root hair development as a useful feature in taxonomic studies; the authors identified species that should be removed from the described tribes and these recommendations were in accordance with the currently accepted phylogenetic classification.Citation23
Another large-scale analysis, this one focused on the root meristem, was published in 2000.Citation25 In this case, the author described the development of root hairs in 3 categories: 1) when trichoblasts derived from the shootward daughter cells after symmetrical divisions (Vp – trichoblasts in vertical patterns and derived from the proximal sister cell; ), 2) when trichoblasts derived from the rootward daughter cells after symmetrical divisions (Vd – trichoblasts in vertical patterns and derived from the distal sister cell), and 3) when trichoblasts were indistinguishable from atrichoblasts (N – no evident trichoblasts). In this approach, the first 2 types (Vp and Vd) correspond to the festucoid type and the third type (N), which has no evident trichoblasts, corresponds to the panicoid type. In this report, 21 species from the Poaceae family (mainly from the Pooideae subfamily) were analyzed.Citation25 However, all of the studies were performed using simple histological stainings, which may generate errors, especially in the interpretation of symmetric and asymmetric divisions. For example, ClowesCitation25 described B. sylvaticum and H. vulgare as type N species with no evident trichoblasts present, whereas studies conducted later, using confocal and electron microscopy, clearly showed that the trichoblasts and atrichoblasts could be distinguished in both species.Citation10,Citation15
In the work of Kim and Dolan,Citation10 techniques including, e.g., confocal laser scanning microscopy and cryo-scanning electron microscopy were used for the root analysis of 2 species—rice (from the Ehrhartoideae subfamily) and B. distachyon (from the Pooideae family). An asymmetrical shootward-last division was observed in rice and after root hair initiation, trichoblasts elongated more slowly than non-root hair cells, whereas in B. distachyon shorter cells localized in rootward position after the asymmetric division and started to develop root hairs.Citation10 Based on their own results and literature data, the authors proposed the hypothesis that the asymmetric division evolved in the Pooideae or in an ancestor of Pooideae because only in this subfamily is the asymmetric division present.Citation10 However, our work on root epidermis differentiation in barley (Pooideae subfamily) clearly indicated that the shootward-last division of root epidermal cells is symmetric and after this division the daughter cells immediately start to elongate asymmetrically.Citation15 Only the shorter cells produce root hairs in the differentiation zone and more than 70% of the shorter epidermal cells are alternately arranged into trichoblasts and atrichoblasts.Citation15 A pattern in which 2 or 3 shorter cells were adjacent to each other was also observed. The differences between trichoblasts and atrichoblasts were visible immediately after cell divisions, before root hair initiation. The shorter cells contained a denser cytoplasm, a greater number of mitochondria and only this type of cells produced root hair tubes.Citation15 Differentiation of both cell types is correlated with the restriction of symplasmic communication between neighboring cells.Citation19,Citation26
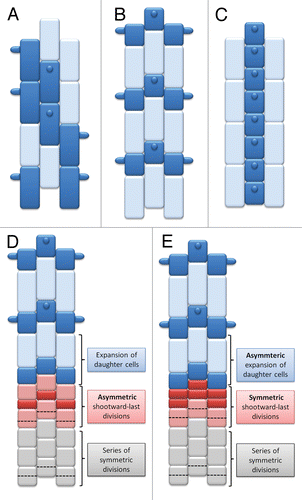
The presented results and literature data indicate that both types of root epidermis differentiation, which result from symmetric and asymmetric divisions, are present among representatives of the Chloridoideae, Pooideae, and Panicoideae subfamilies (; ). However, as was mentioned above, some of the studies that have been performed recently using advanced cell biology techniques, such as confocal laser scanning microscopy or transmission/scanning electron microscopy have shown that several species have been described erroneously.Citation10,Citation15 This is why we postulate the necessity of a more sophisticated analysis of other species and re-examination of plants investigated with the simple microscopy techniques in the future. At present, we suggest using a modified nomenclature for a detail description of root epidermis differentiation (). Type I, when a symmetric shootward-last division is present, corresponding to type B of Sinnott and Bloch,Citation20 the panicoid type of Reeder and von Maltzahn,Citation21 and the N type of Clowes,Citation25 which should be divided into 2 subtypes—IA and IB. In the IA subtype, both daughter cells do not show any morphological differences except for the presence or absence of root hair tubes and any epidermal cell may develop a root hair. The second subtype, IB, should group species in which a symmetric shootward-last division is present, but the differences between daughter cells become visible during differentiation and only shorter cells can produce root hairs, as has been described for rice and barley. Type II, which was originally described by the presence of shorter and longer cells,Citation2 should be defined as a type in which the asymmetric shootward-last division occurs and should also be divided into 2 subtypes. In subtype IIA, corresponding to the Vp type of Clowes,Citation25 a shootward daughter cell produces root hairs after the asymmetric division and IIB, in which only a rootward daughter cell differentiates into a trichoblast (corresponding to the Vd type of Clowes).Citation25 Type III, in which files of trichoblasts and atrichoblasts are present, should remain unchanged (). The proposed classification allows the development of the root epidermis to be described based on the crucial mechanism in this process—the shootward-last division—and subsequently the processes that occur in daughter cells after this division. Now, we know that both asymmetric division and asymmetric cell expansion have been observed in the Chloridoideae, Pooideae, and Panicoideae subfamilies, which indicates that the mechanism of trichoblast/atrichoblast differentiation is not evolutionally conserved across the subfamilies of the Poaceae (; ). However more studies on a larger scale and a re-examination of the literature data that was obtained using simple microscopic techniques are required to describe the evolution of root epidermis patterning in the Poaceae family.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by grants of the Polish National Science Centre: 2011/01/M/NZ2/02979 and 2013/08/T/NZ3/00811.
References
- Cormack RGH. Investigations on the development of root hairs. New Phytol 1935; 34:30 - 54; http://dx.doi.org/10.1111/j.1469-8137.1935.tb06826.x
- Dolan L. Pattern in the root epidermis: an interplay of diffusible signals and cellular geometry. Ann Bot (Lond) 1996; 77:547 - 53; http://dx.doi.org/10.1093/aob/77.6.547
- Dolan L, Costa S. Evolution and genetics of root hair stripes in the root epidermis. J Exp Bot 2001; 52:413 - 7; http://dx.doi.org/10.1093/jexbot/52.suppl_1.413; PMID: 11326047
- Bibikova T, Gilroy S. Root hair development. J Plant Growth Regul 2002; 21:383 - 415; http://dx.doi.org/10.1007/s00344-003-0007-x
- Pemberton LMS, Tsai SL, Lovell PH, Harris PJ. Epidermal patterning in seedling roots of eudicotyledons. Ann Bot (Lond) 2001; 77:547 - 53
- Libault M, Brechenmacher L, Cheng J, Xu D, Stacey G. Root hair systems biology. Trends Plant Sci 2010; 15:641 - 50; http://dx.doi.org/10.1016/j.tplants.2010.08.010; PMID: 20851035
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poethig S, Roberts K. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 1994; 120:2465 - 74
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development 1993; 119:71 - 84; PMID: 8275865
- Datta S, Kim CM, Pernas M, Pires ND, Proust H, Tam T, Vijayakumar P, Dolan L. Root hairs: development, growth and evolution at the plant-soil interface. Plant Soil 2011; 346:1 - 14; http://dx.doi.org/10.1007/s11104-011-0845-4
- Kim CM, Dolan L. Root hair development involves asymmetric cell division in Brachypodium distachyon and symmetric division in Oryza sativa.. New Phytol 2011; 192:601 - 10; http://dx.doi.org/10.1111/j.1469-8137.2011.03839.x; PMID: 21848982
- De Smet I, Beeckman T. Asymmetric cell division in land plants and algae: the driving force for differentiation. Nat Rev Mol Cell Biol 2011; 12:177 - 88; http://dx.doi.org/10.1038/nrm3064; PMID: 21346731
- Paciorek T, Bergmann DC. The secret to life is being different: asymmetric divisions in plant development. Curr Opin Plant Biol 2010; 13:661 - 9; http://dx.doi.org/10.1016/j.pbi.2010.09.016; PMID: 20970370
- Zhang Z, Laux T. The asymmetric division of the Arabidopsis zygote: from cell polarity to an embryo axis. Sex Plant Reprod 2011; 24:161 - 9; http://dx.doi.org/10.1007/s00497-010-0160-x; PMID: 21225434
- Pillitteri LJ, Peterson KM, Horst RJ, Torii KU. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis.. Plant Cell 2011; 23:3260 - 75; http://dx.doi.org/10.1105/tpc.111.088583; PMID: 21963668
- Marzec M, Melzer M, Szarejko I. Asymmetric growth of root epidermal cells is related to the differentiation of root hair cells in Hordeum vulgare (L.). J Exp Bot 2013; 64:5145 - 55; http://dx.doi.org/10.1093/jxb/ert300; PMID: 24043851
- Huang J, Kim CM, Xuan YH, Liu J, Kim TH, Kim BK, Han CD. Formin homology 1 (OsFH1) regulates root-hair elongation in rice (Oryza sativa). Planta 2013; 237:1227 - 39; http://dx.doi.org/10.1007/s00425-013-1838-8; PMID: 23334469
- Huang J, Kim CM, Xuan YH, Park SJ, Piao HL, Je BI, Liu J, Kim TH, Kim BK, Han CD. OsSNDP1, a Sec14-nodulin domain-containing protein, plays a critical role in root hair elongation in rice. Plant Mol Biol 2013; 82:39 - 50; http://dx.doi.org/10.1007/s11103-013-0033-4; PMID: 23456248
- Hochholdinger F, Wen TJ, Zimmermann R, Chimot-Marolle P, da Costa e Silva O, Bruce W, Lamkey KR, Wienand U, Schnable PS. The maize (Zea mays L.) roothairless 3 gene encodes a putative GPI-anchored, monocot-specific, COBRA-like protein that significantly affects grain yield. Plant J 2008; 54:888 - 98; http://dx.doi.org/10.1111/j.1365-313X.2008.03459.x; PMID: 18298667
- Marzec M, Muszynska A, Melzer M, Sas-Nowosielska H, Kurczynska EU. Increased symplasmic permeability in barley root epidermal cells correlates with defects in root hair development. Plant Biol (Stuttg) 2013; •••; http://dx.doi.org/10.1111/plb.12066; PMID: 23927737
- Sinnott EW, Bloch R. Cell polarity and the differentiation of root hairs. Proc Natl Acad Sci U S A 1939; 25:248 - 52; http://dx.doi.org/10.1073/pnas.25.5.248; PMID: 16577890
- Reeder JR, von Maltzahn K. Taxonomic significance of root-hair development in the Gramineae. Proc Natl Acad Sci U S A 1953; 39:593 - 8; http://dx.doi.org/10.1073/pnas.39.7.593; PMID: 16589309
- Row HC, Reeder JR. Root-hair development as evidence of relationships among genera of Gramineae. Am J Bot 1957; 44:596 - 601; http://dx.doi.org/10.2307/2438933
- Soreng RJ, Davidse G, Peterson PM, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Morrone O, Romaschenko K. Catalogue of New World Grasses - A world-wide phylogenetic classification of Poaceae (Gramineae). 2012; http://www.tropicos.org/
- Bloch R. Differentiation in red root-tips of Phalaris arundinacea.. Bull Torrey Bot Club 1943; 70:182 - 3; http://dx.doi.org/10.2307/2481370
- Clowes F. Pattern in root meristem development in angiosperms. New Phytol 2000; 146:83 - 94; http://dx.doi.org/10.1046/j.1469-8137.2000.00614.x
- Marzec M, Kurczynska E. Importance of symplasmic communication in cell differentiation. Plant Signal Behav 2014; 9; http://dx.doi.org/10.4161/psb.27931; PMID: 24476959