Abstract
Plants have to deal with reactive oxygen species (ROS) production, since it could potentially cause severe damages to different cellular components. On the other hand, ROS functioning as important second messengers are implicated in various developmental processes and are transiently produced during biotic or abiotic stresses. Furthermore, the microtubules (MTs) play a primary role in plant development and appear as potent players in sensing stressful situations and in the subsequent cellular responses. Emerging evidence suggests that ROS affect MTs in multiple ways. The cellular redox status seems to be tightly coupled with MTs. ROS signals regulate the organization of tubulin cytoskeleton and induce tubulin modifications. This review aims at summarizing the signaling mechanisms and the key operators orchestrating the crosstalk between ROS and tubulin cytoskeleton in plant cells. The contribution of several molecules, including microtubule associated proteins, oxidases, kinases, phospholipases, and transcription factors, is highlighted.
Reactive oxygen species (ROS) appeared as a consequence of the introduction of oxygen into earth’s atmosphere.Citation1 ROS collectively comprise a group of radical derivatives of oxygen including superoxide anion (·O2-) and hydroxyl radicals (·ΟΗ), or non radicals such as singlet oxygen (1O2) and hydrogen peroxide (H2O2).Citation2 They are extremely reactive molecules that display different properties from molecular oxygen or other chemical species and are capable of inducing severe damages in cellular components. However, due to their unique chemical features, ROS have evolved as essential regulators in various biological processes.Citation1,Citation3 In animal cells, they are produced as byproducts of oxygen metabolism or after enzymatic activity in mitochondria, cytoplasm and extracellular sites.Citation4 In plants, they are generated accidentally or even deliberately in fluctuations in various cellular compartments, such as mitochondria, chloroplasts, peroxisomes as well as the apoplast.Citation3 ROS are critical for pollen tube growth, gametogenesis and embryo development, root hair development, and stomatal function,Citation5-Citation8 and they mediate plant responses to hormonal stimuli.Citation9 Besides their levels rapidly increase during stress resulting in the so-called oxidative burst.Citation10 Hence, ROS generation is of particular importance for the interaction of plants with biotic factors,Citation11 and during abiotic stresses including salt, cold, osmotic, and drought stress.Citation8,Citation10 The crucial contribution of ROS in a broad range of plant developmental and stress processes lies on their ability to act as signals, carry messages, and trigger transduction mechanisms.Citation12
On the other hand, microtubules (MTs) are fundamental organelles of plant cells intimately involved in regulation of their morphogenesisCitation13 and undergo extensive reorganization during abiotic stressCitation14 or biotic interactions.Citation15 Increasing evidence indicates that ROS and MTs are team players and that their fates converge during plant life.Citation16,Citation17 Furthermore, ROS have been repeatedly shown to influence tubulin cytoskeleton in various ways.Citation18 This review attempts to summarize the existing information relevant to ROS and MTs in plants, trying to shed some light on their emerging crosstalk.
ROS homeostasis and tubulin cytoskeleton during plant life
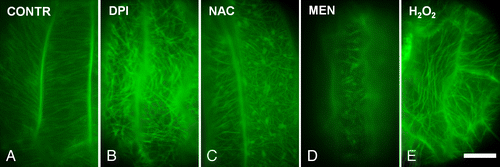
ROS oscillations occur along with fluctuations of antioxidants throughout plant cell division.Citation19,Citation20 Apart from its role in regulating specific cell cycle checkpoints,Citation19 ROS homeostasis, namely the balance between ROS generation and scavenging,Citation1,Citation10 is critical for proper completion of cell division due to its interference with mechanisms that direct the organization of successive MT arrays in dividing cells.Citation17,Citation18 The chemically-induced disturbance of ROS homeostasis, assessed by using selective ROS-sensitive fluorescent probes, severely affects the MT arrays. Oxidative stress induced by hexavalent chromiumCitation21 or other chemicalsCitation17,Citation18 disrupts mitosis and cytokinesis in root-tip cells of angiosperms. The elimination of cellular ROS levels results in almost the same effects. Diphenylene iodonium, a specific inhibitor of ROS-producing NADPH oxidase and the ROS scavenger n-acetyl-cysteine harms mitotic and cytokinetic MT arrays in Triticum turgidum and Arabidopsis thaliana root tips.Citation18 What’s more, many cells of rhd2 A. thaliana mutant lacking the function of a NADPH oxidase display low ROS levels in roots and simultaneously defects on MT systems.Citation17,Citation18 In addition, exogenous application of the antioxidant tripeptide glutathione on Picea abies cells affects interphase cortical MTs and the MT-preprophase band.Citation22 The effects of ROS imbalance on MTs are not restricted to dividing cells. Treatments of GFP:TUA6 transgenic Arabidopsis seedlings with ROS modulators revealed that the cortical MTs in developing epidermal cells of hypocotyls and cotyledons are also sensitive to alterations in ROS levels (). Moreover, Arabidopsis epidermal cells exposure to ROS-generating titanium dioxide (TiO2) nanoparticles disrupted the MT networks and led to formation of tubulin aggregates.Citation23 Hence, either ROS overproduction or elimination forces the disappearance of MTs and their replacement by stable and resistant atypical tubulin polymers (see also ref. Citation24). These polymers may be macrotubules i.e., tubulin tubules displaying higher outer diameter compared with typical MTs, or paracrystal conformations containing tubulin referred as tubulin paracrystals.Citation18
Figure 1. The disturbance of reactive oxygen species (ROS) homeostasis affects tubulin cytoskeleton organization in transgenic Arabidopsis plants expressing GFP:TUA6. (A-E): Epidermal cells from 4-d-old hypocotyls of seedlings treated with distilled water (A) or with ROS modulators (B-E). The decrease of ROS levels forced either by the NADPH oxidase inhibitor diphenylene iodonium (DPI) or by the ROS scavenger n-acetyl-cysteine (NAC) results in disruption and reorganization of the tubulin cytoskeleton into random mesh-like arrays (B, C; cf. A). Similarly, oxidative stress induced by the oxidizing agent menadione (MEN) and hydrogen peroxide (Η2Ο2) results in MT remodeling (D, Ε; cf. A). CONTR, distilled water; DPI, 50 μΜ; ΝΑC, 500 μΜ; ΜΕΝ, 50 μΜ, Η2Ο2, 5 mM. Treatments: 2 h. Scale bar: 10 μm.
On the other hand, elevation of ROS levels has been recorded during almost every type of biotic or abiotic stress and functions as a universal signal triggering downstream responses.Citation25 Stress-induced ROS generation occurs in different cellular sources, activates mitogen-activated protein kinase (MAPK) cascades and leads to upregulation of several transcription factors.Citation26 In addition, many different kinds of stress induce rearrangements in tubulin cytoskeleton.Citation14,Citation15 MTs participate in biotic stress responses, including the pathogen attack-induced programmed cell death.Citation27 Likewise, disruption and depolymerization of MTs at the site of pathogen infection has been repeatedly observed.Citation15,Citation27 Moreover, MT reorganization occurs along with oxidative burst during mechanical stress and both these phenomena are implicated in sensing mechanical stimulation.Citation28,Citation29 Therefore, it is reasonable to assume that ROS generation and cytoskeletal alterations might be coupled during plant life. Both drive developmental procedures and, during stress, they promote the perception of the stimuli, as well as the consequent responses.
Implications of cytoskeletal contribution to deliberate ROS production
One of the most important protein groups that respond to stress and concurrently contribute to ROS production is that of Rbohs (respiratory burst oxidase homologs).Citation10 They are plasmalemmal enzymes that catalyze superoxide production in the apoplast.Citation30 Superoxide may be protonated to form hydroperoxyl radical (·O2H) or converted to hydrogen peroxide, which easily diffuses into the cytosol and initiates signaling transduction.Citation30 The activation of NAPDH oxidases followed by ROS generation has been recorded in defense responses of A. thaliana against Verticillum dahliae toxinsCitation31 or those against the bacterial elicitors flg22 and Harpin in Vitis cells.Citation32 Using diphenylene iodonium alone or together with exogenously added hydrogen peroxide Yao et al.Citation31 showed that the NADPH oxidase-mediated ROS accumulation is responsible for MT-depolymerization. In turn, the depolymerization of cortical MTs has been repeatedly suggested to be essential for the expression of specific resistant genes.Citation31,Citation32
Recent evidence suggested the existence of a positive feedback relationship between tubulin cytoskeleton and ROS production and revealed a new mode of NADPH oxidase regulation. In this relationship, the MT-associated protein MAP65–1 displays a unique role, different than usual. ZmMAP65–1a that was found to be increased after application of hydrogen peroxide was also shown to regulate hydrogen peroxide amplification upon treatment of Zea mays leaves with brassinosteroid phytohormones.Citation33 The ROS-responsive ZmMPK5 interacts with ZmMAP65–1a toward activating the expression of NADPH oxidase genes and the subsequent responses.Citation33 In particular, the transcription of several antioxidant defense genes, which is initiated after NADPH oxidase activation, requires the phosphorylation of ZmMAP65–1a by ZmMPK5.Citation33,Citation34 Therefore, it is very interesting that, during stress, cytoskeletal proteins respond to elevated ROS levels and at the same time activate the NADPH oxidase assisting the purposeful production of ROS.
The implication of NADPH oxidase and ROS homeostasis in regulation of MT organization is further supported by the case of rhd2 A. thaliana mutants, which lack one of the A. thaliana NADPH oxidases. The rhd2 mutants display low ROS levels and simultaneously tubulin cytoskeleton defects and a significant amount of macrotubules in roots.Citation18 Interestingly, these plants exhibit extremely elevated levels of a p46 p38-like MAPK.Citation35 It was recently found that in angiosperms the p46 MAPK is implicated in sensing cellular ROS level alterations. ROS overproduction as well as elimination of ROS levels resulted in the activation of this protein.Citation35 Once activated, p46 drives the assembly of atypical tubulin polymers.Citation35 This strengthens the view that the mechanisms maintaining homeostasis during low or elevated ROS levels are connected by feedback loops and tightly linked to cytoskeletal responses.
ROS signaling and the role of phosphatidic acid
The p46 MAPK is assumed to be directly or indirectly involved in MAP65–1 activity toward the assembly of atypical tubulin polymers during ROS imbalance.Citation35 Moreover, MAP65–1 is phosphorylated by several other ROS-responsive MAPKs. AtMPK3, AtMPK4, and AtMPK6, which are activated by ROS,Citation36 have been shown to directly phosphorylate AtMAP65–1.Citation37,Citation38 The activity of MAP65–1 is also modulated by its interaction with phosphatidic acid (PA), an important lipid mediator produced by phospholipase D (PLD).Citation39 PA and MAP65–1 synergy plays a critical role during salt stress, since PA binds to MAP65–1 and enhances tubulin polymerization and the MT-bundling activity of MAP65–1.Citation39 Salinity forces MT depolymerization followed by MT reorganization into stable arrays, responses leading to salt tolerance. The oxidative burst seems to have a significant part in these processes.Citation24 PA production and activation of p46 p38-like MAPK mediate the assembly of macrotubules during protoplast volume regulation in plasmolysed T. turgidum root-tip cells.Citation40 In A. thaliana, PA produced by PLD regulates ROS generation by NADPH oxidase during stomatal closure induced by treatment with abscisic acid.Citation41 Interestingly, in Vicia a p38-like MAPK participates in abscisic acid triggered oxidative burst, whereas this hormone was found to activate p38 MAPK in chloronema cells of Funaria hygrometrica.Citation42,Citation43 Considering the data presented above, it may be proposed that the interactions between PLD and its derivative PA, p38-like MAPKs and NAPDH oxidase-derived ROS collectively orchestrate the rearrangements of tubulin cytoskeleton, which are part of the responses to external stimuli.
This implies that ROS mediate stress responses and hormone signaling, through converging pathways. Such responses repeatedly include participation of the tubulin cytoskeleton and recruitment of tubulin associated proteins. In this direction, ZmMPK5 is activated by abscisic acidCitation44 and concurrently, as it was previously noticed, interacts with ZmMAP65–1a toward ROS production after treatment with brassinosteroids.Citation33 Abscisic acid also strongly modifies the levels of α and β tubulin mRNA transcriptsCitation45 and induces depolymerization and reorganization of cortical MTs.Citation46,Citation47 In addition, it is known that the rest classes of phytohormones also modulate ROS levels in plants,Citation9 whereas almost all the hormones interfere with plant cytoskeleton.Citation48,Citation49 Although, some implications suggest ROS involvement in hormone- induced tubulin cytoskeleton remodeling, their exact role cannot be easily identified.
MT-associated transcription factors involved in fine tuning of ROS levels
An attractive example of a possible interconnection between MTs, hormones and effector molecules are DELLA proteins. These proteins are transcription factors, which participate in hormone signaling mechanismsCitation50 and mediate several developmental processes in plants.Citation51 During stress, DELLA proteins promote the transcription of genes encoding antioxidant proteins, such as superoxide dismutase. Hence, they contribute to fine tuning of cellular ROS levels.Citation50,Citation52 Importantly, DELLA proteins have been recently implicated in governing cortical MT organization in Arabidopsis during cell expansion induced by gibberellins.Citation51 In particular, these proteins affect, among others, the availability of α/β tubulin heterodimers and consequently the polymerization of MTs due to their ability to direct the prefoldin complex localization, which is required for tubulin folding. Furthermore, Locascio et al.Citation51 note that the DELLA-dependent accumulation of prefoldin complex in nucleus is potentially crucial for the expression of several tubulin encoding genes. This is very interesting since it is known that the disturbance of ROS homeostasis affects the levels of α or β tubulin in plants and animals, leading in some cases to elevated tubulin levels (see also ref. Citation23).Citation17,Citation53 We may then extrapolate that the upregulation of tubulin is part of the antioxidant defense responses induced by transcriptional mediators like DELLA proteins. In this direction, tubulin upregulation may follow treatments with chemicals inducing low ROS levels.Citation17 These data further support the hypothesis that the dynamic regulation of tubulin cytoskeleton is part of the cellular mechanisms driving the restoration of ROS homeostasis.
Some aspects on the oxidation of tubulins
Paradigms from animal cells support the view that the regulation of tubulin cytoskeleton act protectively against pertubations of cellular redox state.Citation54 It is well known that tubulins are extremely sensitive to oxidative stress and that their cysteine residues could be easily oxidized, even after a short exposure to ROS.Citation54 Oxidation of cysteine sulfhydryl groups may promote the cross-linking between α and β tubulin due to formation of disulfide bonds, thus preventing the assembly of MTs.Citation54,Citation55 Of outstanding interest is the case of βIII isoform of animal β tubulins (reviewed by LudueñaCitation54). This isoform lacks cys239, one of the cysteines of β tubulin, which easily forms disulfide bonds and its oxidation has been straightly correlated with the inhibition of MT assembly.Citation54 Importantly, the βΙΙΙ isoform is present at high levels in cells displaying regularly elevated ROS levels and is upregulated in oxidative stress treated cells.Citation54 Therefore, it seems that redox sensing may be related to the expression of different tubulin isoforms and this may prevent cellular damage.
Such information regarding plant cell tubulins is missing. However, proteome examination of Α. thaliana cells treated with hydrogen peroxide revealed that α and β plant tubulins are also susceptible to oxidation.Citation56 Besides, using proteomic analysis it was found that both α and β tubulins undergo S-glutathionylation in A. thaliana cells, after application of the oxidant t-butyl-hydroperoxide (t-BuOOH).Citation57 Glutathione binding offers protection to thiol groups of cysteine against oxidation. Nevertheless, thiolation itself could influence the activity of the modified protein or even drive signal transduction.Citation57,Citation58 Thus, it seems that apart from mechanisms that perceive cellular redox status, additional regulatory mechanisms sense the redox status of tubulin. Since the later is strongly related to the capability for MT assembly,Citation59,Citation60 such mechanisms may protect tubulin against irreversible oxidation.Citation60
Tubulin cytoskeleton remodeling during ROS imbalance
The formation of stable atypical tubulin polymers, following the disturbance of ROS homeostasis, can be explained considering that they contain or consist of modified tubulins. Apart from oxidative modifications ROS imbalance was found to rapidly induce other post-translational modifications in plants, such as tubulin acetylationCitation17,Citation18 (see also ref. Citation21) or slight alterations of the tyrosinated tubulin levels.Citation17 Such modifications that amend the tubulin polymer properties are common in stress conditions.Citation32 For example, hyperosmotic treatment stimulates phosphorylation of α-tubulin.Citation61 Interestingly, osmotic stress results not only in cellular oxidative burst,Citation10 but also in disappearance of MTs followed by the assembly of macrotubules.Citation40 ROS production and its interplay with MT reorganization allow adaptive response during osmotic stress in a model proposed by Nick et al.Citation62 Taking into consideration the data presented above, we may propose that tubulin post-translational modifications during ROS homeostasis disturbance could be correlated with the behavior of the tubulin cytoskeleton. A possible explanation of this behavior might be that such modifications probably, among others, influence the interaction of tubulins or MTs with associated macromolecules (see also ref. Citation63) as well as the properties of tubulin polymers.
Regarding the process of MT destruction, one would suggest that ROS might directly or indirectly interfere with aspects of proper tubulin polymerization, such as the concentration of calcium or the availability of free tubulin heterodimers.Citation64 It is known that increased ROS levels are implicated in raising calcium levelsCitation7 and that MTs are sensitive to calcium concentrations.Citation64 Elevated calcium may lead to destruction of MTsCitation65 and ROS might interfere with the amount of tubulin available for polymerization.Citation17 The disruption of MTs could potentially be mediated by the activation of MT severing proteins like katanin. Katanin is a heterodimeric protein that binds to and cleaves MTs.Citation66 In plants, it was shown to be recruited during cytoskeletal responses to several stimuli that induce elevation of cellular ROS levels, such as mechanical stress and wounding,Citation8,Citation28,Citation29 blue light,Citation67,Citation68 and hormonal responses.Citation9,Citation66 However, the extent of association of ROS fluctuations with katanin activation remains to be found. Besides, MT disappearance could be further promoted by weakening of MAP binding to MTs. For instance, phosphorylation of MAP65–1, which belongs to MAP65 protein family and has been implicated in MT bundling, results in its dissociation from MTs and leads to MT disassembly.Citation37 Therefore, it is very interesting that MAP65–1 is one of the substrates of the ROS-activated MAPKs, AtMPK3, AtMPK4, AtMPK6.Citation36-Citation38
Importantly, MAP65–1 mediates the formation of atypical tubulin polymers, i.e., macrotubules and tubulin paracrystals during ROS disturbance.Citation18 MAP65 proteins also participate in the assembly of tubulin paracrystals after treatment of Vigna sinensis roots with colchicine.Citation69 What’s more, the MAP65–6 along with MAP65–1 has been found to promote the organization of resistant thick MT bundles during salt stress, which appear as a mesh-like network.Citation70 The binding capacity of different MAP65 proteins to MTs highly depends on the MT-binding domains of MAP65 isotypes, which are localized to the C-terminal of each protein molecule.Citation71 Besides, post-translational modifications of tubulin could modulate the binding potential to different MAPs.Citation63 Hence, we may suggest that, during atypical tubulin polymer formation accompanying loss of ROS homeostasis, MAPs promoting the assembly of tubulin polymers other than MTs preferably bind to modified tubulin.
Concluding Remarks
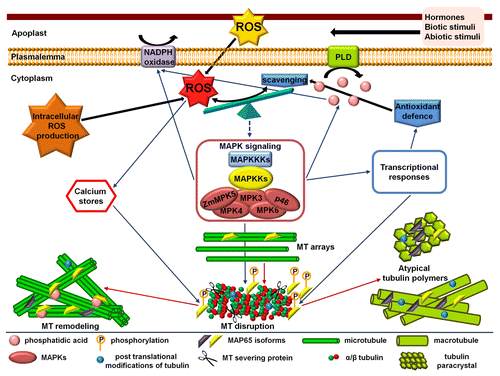
ROS homeostasis is of particular importance for plant life and, nowadays, emerges as a significant regulator of tubulin cytoskeleton. The interplay between ROS and tubulin cytoskeleton is implicated in sensing almost every stressful situation and drives the succeeding responses. The responses against various stimuli are mediated through converging signaling cascades that aim at restoring ROS homeostasis. During this process the remodeling of tubulin cytoskeleton seems to play a crucial role. Such remodeling includes, among others, the recruitment of MAPs which is forced by MAPK signaling and regulatory mechanisms acting at transcriptional and post-transcriptional level. Some of the possible participants of this complex crosstalk, which were discussed in the present article, are illustrated in . The existence of other signaling routes, protein components and additional mechanisms cannot be excluded. Further experimentation is needed in order to establish a more clear relationship between ROS homeostasis and tubulin cytoskeleton. ROS appear as ideal players in fine tuning of the behavior of tubulin cytoskeleton during stress. It would therefore be very promising to further investigate the ROS-dependent MT-mediated developmental processes.
Figure 2. Simplified hypothetical model describing the changes mediated by reactive oxygen species (ROS) level alteration with respect to the organization of tubulin cytoskeleton. ROS and antioxidant balance is further influenced by abiotic or biotic stimuli. In any case, the ROS produced in the apoplast (e.g., derived from NADPH oxidase) enter the cytosol and together with intracellularly generated ROS constitute the total amount of cellular ROS. Consequently, the result of the tag war between oxidant generation and antioxidant defenses is perceived and either directly or through feedback connected signaling cascades forces the reorganization of tubulin cytoskeleton. The reorganization among others aids the restoration of ROS homeostasis. The microtubule (MT) arrays disappear and MT remodeling, or the assembly of atypical tubulin polymers like macrotubules and tubulin paracrystals, follows. These processes are mediated by MT-associated proteins (MAPs) assisted by other molecules such as phosphatidic acid produced by phospholipase D (PLD). The modifications of tubulin or MAPs at the post-translational level may crucially contribute to this interplay.
| Abbreviations: | ||
| MAP | = | microtubule associated protein |
| MAPK | = | mitogen-activated protein kinase |
| MT | = | microtubule |
| PA | = | phosphatidic acid |
| PLD | = | phospholipase D |
| ROS | = | reactive oxygen species |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to express their thanks to Dr František Baluška (University of Bonn, Germany) for his kind invitation to prepare this review and Dr Ioannis-Dimosthenis Adamakis (Aristotle University of Thessaloniki, Greece) for the GFP:TUA6 A. thaliana seeds. This work has been financed by the University of Athens, Greece.
References
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci 2004; 9:490 - 8; http://dx.doi.org/10.1016/j.tplants.2004.08.009; PMID: 15465684
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 2006; 141:312 - 22; http://dx.doi.org/10.1104/pp.106.077073; PMID: 16760481
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 2004; 55:373 - 99; http://dx.doi.org/10.1146/annurev.arplant.55.031903.141701; PMID: 15377225
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 2011; 194:7 - 15; http://dx.doi.org/10.1083/jcb.201102095; PMID: 21746850
- Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 2007; 174:742 - 51; http://dx.doi.org/10.1111/j.1469-8137.2007.02042.x; PMID: 17504458
- Martin MV, Fiol DF, Sundaresan V, Zabaleta EJ, Pagnussat GC. oiwa, a female gametophytic mutant impaired in a mitochondrial manganese-superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 2013; 25:1573 - 91; http://dx.doi.org/10.1105/tpc.113.109306; PMID: 23653473
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003; 422:442 - 6; http://dx.doi.org/10.1038/nature01485; PMID: 12660786
- Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxid Redox Signal 2006; 8:1757 - 64; http://dx.doi.org/10.1089/ars.2006.8.1757; PMID: 16987029
- Kwak JM, Nguyen V, Schroeder JI. The role of reactive oxygen species in hormonal responses. Plant Physiol 2006; 141:323 - 9; http://dx.doi.org/10.1104/pp.106.079004; PMID: 16760482
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 2010; 33:453 - 67; http://dx.doi.org/10.1111/j.1365-3040.2009.02041.x; PMID: 19712065
- Torres MA. ROS in biotic interactions. Physiol Plant 2010; 138:414 - 29; http://dx.doi.org/10.1111/j.1399-3054.2009.01326.x; PMID: 20002601
- Møller IM, Sweetlove LJ. ROS signalling-specificity is required. Trends Plant Sci 2010; 15:370 - 4; http://dx.doi.org/10.1016/j.tplants.2010.04.008; PMID: 20605736
- Kost B, Mathur J, Chua N-H. Cytoskeleton in plant development. Curr Opin Plant Biol 1999; 2:462 - 70; http://dx.doi.org/10.1016/S1369-5266(99)00024-2; PMID: 10607658
- Nick P. Microtubules, signalling and abiotic stress. Plant J 2013; 75:309 - 23; http://dx.doi.org/10.1111/tpj.12102; PMID: 23311499
- Hardham AR. Microtubules and biotic interactions. Plant J 2013; 75:278 - 89; http://dx.doi.org/10.1111/tpj.12171; PMID: 23480445
- Potters G, Pasternak TP, Guisez Y, Jansen MAK. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ 2009; 32:158 - 69; http://dx.doi.org/10.1111/j.1365-3040.2008.01908.x; PMID: 19021890
- Livanos P, Apostolakos P, Galatis B. Plant cell division: ROS homeostasis is required. Plant Signal Behav 2012; 7:771 - 8; http://dx.doi.org/10.4161/psb.20530; PMID: 22751303
- Livanos P, Galatis B, Quader H, Apostolakos P. Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana.. Cytoskeleton (Hoboken) 2012; 69:1 - 21; http://dx.doi.org/10.1002/cm.20538; PMID: 21976360
- Potters G, De Gara L, Asard H, Horemans N. Ascorbate and glutathione: guardians of the cell cycle, partners in crime?. Plant Physiol Biochem 2002; 40:537 - 48; http://dx.doi.org/10.1016/S0981-9428(02)01414-6
- Vivancos PD, Dong Y, Ziegler K, Markovic J, Pallardó FV, Pellny TK, Verrier PJ, Foyer CH. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J 2010; 64:825 - 38; http://dx.doi.org/10.1111/j.1365-313X.2010.04371.x; PMID: 21105929
- Eleftheriou EP, Adamakis I-DS, Fatsiou M, Panteris E. Hexavalent chromium disrupts mitosis by stabilizing microtubules in Lens culinaris root tip cells. Physiol Plant 2013; 147:169 - 80; http://dx.doi.org/10.1111/j.1399-3054.2012.01652.x; PMID: 22607451
- Urbanek A, Zechmann B, Zellnig G, Müller M. Aspects of glutathione treatment on the cytoskeleton in different cells of Picea abies.. Phyton 2003; 43:319 - 33
- Wang S, Kurepa J, Smalle JA. Ultra-small TiO(2) nanoparticles disrupt microtubular networks in Arabidopsis thaliana.. Plant Cell Environ 2011; 34:811 - 20; http://dx.doi.org/10.1111/j.1365-3040.2011.02284.x; PMID: 21276012
- Wang C, Li J, Yuan M. Salt tolerance requires cortical microtubule reorganization in Arabidopsis. Plant Cell Physiol 2007; 48:1534 - 47; http://dx.doi.org/10.1093/pcp/pcm123; PMID: 17906320
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 2006; 9:436 - 42; http://dx.doi.org/10.1016/j.pbi.2006.05.014; PMID: 16759898
- Jaspers P, Kangasjärvi J. Reactive oxygen species in abiotic stress signaling. Physiol Plant 2010; 138:405 - 13; http://dx.doi.org/10.1111/j.1399-3054.2009.01321.x; PMID: 20028478
- Smertenko A, Franklin-Tong VE. Organisation and regulation of the cytoskeleton in plant programmed cell death. Cell Death Differ 2011; 18:1263 - 70; http://dx.doi.org/10.1038/cdd.2011.39; PMID: 21566662
- Monshausen GB, Gilroy S. Feeling green: mechanosensing in plants. Trends Cell Biol 2009; 19:228 - 35; http://dx.doi.org/10.1016/j.tcb.2009.02.005; PMID: 19342240
- Landrein B, Hamant O. How mechanical stress controls microtubule behavior and morphogenesis in plants: history, experiments and revisited theories. Plant J 2013; 75:324 - 38; http://dx.doi.org/10.1111/tpj.12188; PMID: 23551516
- Fluhr R. Reactive oxygen-generating NADPH oxidases in plants. In: Reactive oxygen species in plant signaling, signaling and communication in plants. del Ryo LA, Puppo A, eds. Heidelberg: Spinger-Verlag, 2009; 1-23.
- Yao L-L, Zhou Q, Pei B-L, Li Y-Z. Hydrogen peroxide modulates the dynamic microtubule cytoskeleton during the defence responses to Verticillium dahliae toxins in Arabidopsis.. Plant Cell Environ 2011; 34:1586 - 98; http://dx.doi.org/10.1111/j.1365-3040.2011.02356.x; PMID: 21707649
- Chang X, Nick P. Defence signalling triggered by Flg22 and Harpin is integrated into a different stilbene output in Vitis cells. PLoS One 2012; 7:e40446; http://dx.doi.org/10.1371/journal.pone.0040446; PMID: 22792328
- Zhu Y, Zuo M, Liang Y, Jiang M, Zhang J, Scheller HV, Tan M, Zhang A. MAP65-1a positively regulates H2O2 amplification and enhances brassinosteroid-induced antioxidant defence in maize. J Exp Bot 2013; 64:3787 - 802; http://dx.doi.org/10.1093/jxb/ert215; PMID: 23956414
- Zhang A, Zhang J, Ye N, Cao J, Tan M, Zhang J, Jiang M. ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. J Exp Bot 2010; 61:4399 - 411; http://dx.doi.org/10.1093/jxb/erq243; PMID: 20693409
- Livanos P, Galatis B, Gaitanaki C, Apostolakos P. Phosphorylation of a p38-like MAPK is involved in sensing cellular redox state and drives atypical tubulin polymer assembly in angiosperms. Plant Cell Environ 2013; •••; http://dx.doi.org/10.1111/pce.12222; PMID: 24138172
- Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 2010; 61:621 - 49; http://dx.doi.org/10.1146/annurev-arplant-042809-112252; PMID: 20441529
- Smertenko AP, Chang H-Y, Sonobe S, Fenyk SI, Weingartner M, Bögre L, Hussey PJ. Control of the AtMAP65-1 interaction with microtubules through the cell cycle. J Cell Sci 2006; 119:3227 - 37; http://dx.doi.org/10.1242/jcs.03051; PMID: 16847052
- Hoehenwarter W, Thomas M, Nukarinen E, Egelhofer V, Röhrig H, Weckwerth W, Conrath U, Beckers GJ. Identification of novel in vivo MAP kinase substrates in Arabidopsis thaliana through use of tandem metal oxide affinity chromatography. Mol Cell Proteomics 2013; 12:369 - 80; http://dx.doi.org/10.1074/mcp.M112.020560; PMID: 23172892
- Zhang Q, Lin F, Mao T, Nie J, Yan M, Yuan M, Zhang W. Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis.. Plant Cell 2012; 24:4555 - 76; http://dx.doi.org/10.1105/tpc.112.104182; PMID: 23150630
- Komis G, Quader H, Galatis B, Apostolakos P. Macrotubule-dependent protoplast volume regulation in plasmolysed root-tip cells of Triticum turgidum: involvement of phospholipase D. New Phytol 2006; 171:737 - 50; http://dx.doi.org/10.1111/j.1469-8137.2006.01784.x; PMID: 16918545
- Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X. Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009; 21:2357 - 77; http://dx.doi.org/10.1105/tpc.108.062992; PMID: 19690149
- Jiang J, Song C-P. MEK1/2 and p38-like MAP kinase successively mediate H(2)O(2) signaling in Vicia guard cell. Plant Signal Behav 2008; 3:996 - 8; PMID: 19704432
- D’Souza JS, Johri MM. ABA and NaCl activate myelin basic kinase in the chloronema cells of the moss Funaria hygrometrica.. Plant Physiol Biochem 2002; 40:17 - 24; http://dx.doi.org/10.1016/S0981-9428(01)01344-4
- Liu Y. Roles of mitogen-activated protein kinase cascades in ABA signaling. Plant Cell Rep 2012; 31:1 - 12; http://dx.doi.org/10.1007/s00299-011-1130-y; PMID: 21870109
- Gianì S, Qin X, Faoro F, Breviario D. In rice, Oryzalin and abscisic acid differentially affect tubulin mRNA and protein levels. Planta 1998; 205:334 - 41; http://dx.doi.org/10.1007/s004250050328; PMID: 9640661
- Jiang C-J, Nakajima N, Kondo N. Disruption of microtubules by abscisic acid in guard cells of Vicia faba L. Plant Cell Physiol 1996; 37:697 - 701; http://dx.doi.org/10.1093/oxfordjournals.pcp.a029001
- Shibaoka H. Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol 1994; 45:527 - 44; http://dx.doi.org/10.1146/annurev.pp.45.060194.002523
- Baluška F, Volkmann D, Barlow PW. Hormone-cytoskeleton interactions in plant cells. In: Biochemistry and molecular biology of plant hormones. Hooykaas PJJ, Hall MA, Libbenga KR, eds. Amsterdam: Elsevier, 1999; 363-90.
- Blume YB, Krasylenko YA, Yemets AI. Effects of phytohormones on the cytoskeleton of the plant cell. Russ J Plant Physiol 2012; 59:515 - 29; http://dx.doi.org/10.1134/S1021443712040036
- Tognetti VB, Mühlenbock P, Van Breusegem F. Stress homeostasis - the redox and auxin perspective. Plant Cell Environ 2012; 35:321 - 33; http://dx.doi.org/10.1111/j.1365-3040.2011.02324.x; PMID: 21443606
- Locascio A, Blázquez MA, Alabadí D. Dynamic regulation of cortical microtubule organization through prefoldin-DELLA interaction. Curr Biol 2013; 23:804 - 9; http://dx.doi.org/10.1016/j.cub.2013.03.053; PMID: 23583555
- Achard P, Renou J-P, Berthomé R, Harberd NP, Genschik P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 2008; 18:656 - 60; http://dx.doi.org/10.1016/j.cub.2008.04.034; PMID: 18450450
- Santa-María I, Smith MA, Perry G, Hernández F, Avila J, Moreno FJ. Effect of quinones on microtubule polymerization: a link between oxidative stress and cytoskeletal alterations in Alzheimer’s disease. Biochim Biophys Acta 2005; 1740:472 - 80; http://dx.doi.org/10.1016/j.bbadis.2004.11.024; PMID: 15949717
- Ludueña RF. A hypothesis on the origin and evolution of tubulin. Int Rev Cell Mol Biol 2013; 302:41 - 185; http://dx.doi.org/10.1016/B978-0-12-407699-0.00002-9; PMID: 23351710
- Landino LM, Hasan R, McGaw A, Cooley S, Smith AW, Masselam K, Kim G. Peroxynitrite oxidation of tubulin sulfhydryls inhibits microtubule polymerization. Arch Biochem Biophys 2002; 398:213 - 20; http://dx.doi.org/10.1006/abbi.2001.2729; PMID: 11831852
- Wang H, Wang S, Lu Y, Alvarez S, Hicks LM, Ge X, Xia Y. Proteomic analysis of early-responsive redox-sensitive proteins in Arabidopsis.. J Proteome Res 2012; 11:412 - 24; http://dx.doi.org/10.1021/pr200918f; PMID: 22050424
- Dixon DP, Skipsey M, Grundy NM, Edwards R. Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol 2005; 138:2233 - 44; http://dx.doi.org/10.1104/pp.104.058917; PMID: 16055689
- Rouhier N, Lemaire SD, Jacquot J-P. The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 2008; 59:143 - 66; http://dx.doi.org/10.1146/annurev.arplant.59.032607.092811; PMID: 18444899
- Clark HM, Hagedorn TD, Landino LM. Hypothiocyanous acid oxidation of tubulin cysteines inhibits microtubule polymerization. Arch Biochem Biophys 2014; 541:67 - 73; http://dx.doi.org/10.1016/j.abb.2013.10.026; PMID: 24215946
- Landino LM, Moynihan KL, Todd JV, Kennett KL. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun 2004; 314:555 - 60; http://dx.doi.org/10.1016/j.bbrc.2003.12.126; PMID: 14733943
- Ban Y, Kobayashi Y, Hara T, Hamada T, Hashimoto T, Takeda S, Hattori T. α-tubulin is rapidly phosphorylated in response to hyperosmotic stress in rice and Arabidopsis. Plant Cell Physiol 2013; 54:848 - 58; http://dx.doi.org/10.1093/pcp/pct065; PMID: 23628996
- Nick P. Why to spend tax money on plant microtubules? In: Applied Plant Cell Biology. Nick P, Opatrný Z. eds. Plant Cell Monographs. Berlin: Springer-Verlag, 2014; 22:39-67.
- Cai G. Assembly and disassembly of plant microtubules: tubulin modifications and binding to MAPs. J Exp Bot 2010; 61:623 - 6; http://dx.doi.org/10.1093/jxb/erp395; PMID: 20080825
- Fosket DE, Morejohn LC. Structural and functional organization of tubulin. Annu Rev Plant Physiol Plant Mol Biol 1992; 43:201 - 40; http://dx.doi.org/10.1146/annurev.pp.43.060192.001221
- Hepler PK, Hush JM. Behavior of microtubules in living plant cells. Plant Physiol 1996; 112:455 - 61; PMID: 12226402
- Burk DH, Zhong R, Ye Z-H. The katanin microtubule severing protein in plants. J Integr Plant Biol 2007; 49:1174 - 82; http://dx.doi.org/10.1111/j.1672-9072.2007.00544.x
- Lindeboom JJ, Nakamura M, Hibbel A, Shundyak K, Gutierrez R, Ketelaar T, Emons AM, Mulder BM, Kirik V, Ehrhardt DW. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 2013; 342:1245533; http://dx.doi.org/10.1126/science.1245533; PMID: 24200811
- Wen F, Xing D, Zhang L. Hydrogen peroxide is involved in high blue light-induced chloroplast avoidance movements in Arabidopsis.. J Exp Bot 2008; 59:2891 - 901; http://dx.doi.org/10.1093/jxb/ern147; PMID: 18550599
- Panteris E, Komis G, Adamakis I-DS, Šamaj J, Bosabalidis AM. MAP65 in tubulin/colchicine paracrystals of Vigna sinensis root cells: possible role in the assembly and stabilization of atypical tubulin polymers. Cytoskeleton (Hoboken) 2010; 67:152 - 60; PMID: 20217678
- Mao T, Jin L, Li H, Liu B, Yuan M. Two microtubule-associated proteins of the Arabidopsis MAP65 family function differently on microtubules. Plant Physiol 2005; 138:654 - 62; http://dx.doi.org/10.1104/pp.104.052456; PMID: 15908607
- Smertenko AP, Kaloriti D, Chang H-Y, Fiserova J, Opatrny Z, Hussey PJ. The C-terminal variable region specifies the dynamic properties of Arabidopsis microtubule-associated protein MAP65 isotypes. Plant Cell 2008; 20:3346 - 58; http://dx.doi.org/10.1105/tpc.108.063362; PMID: 19060108
