Abstract
Establishment of proper polarities along the adaxial-abaxial, proximodistal, and medial-lateral axes is a critical step for the expansion of leaves from leaf primordia. It has been shown that the MYB domain protein, ASYMMETRIC LEAVES1/ROUGH SHEATH2/PHANTASTICA (collectively named ARP) plays an important role in this process. Loss of function of ARP leads to severe leaf polarity defects, such as abaxialized or needle-like leaves. In addition to its role in leaf polarity establishment, we have recently shown that the Medicago truncatula ARP gene, MtPHAN, also plays a role in leaf petiole identity regulation. We show that a mutation of MtPHAN results in petioles acquiring characteristics of the motor organ, pulvinus, including small epidermal cells with extensive cell surface modifications and altered vascular tissue development. Taken together, our results reveal a previously unidentified function of ARP in leaf development.
Plant leaf can be broadly categorized as being simple or compound, based on the complexity of leaves. For example, a simple leaf consists of a single undivided blade; whereas a compound leaf is made of multiple unites of blade, known as leaflets, each resembling a simple leaf. Leaf primordia that lead to either simple or compound leaves initiate from the flanks of the shoot apical meristem (SAM) and expand along the adaxial-abaxial, proximodistal, and mediolateral axes to develop fully-expanded leaves. It is known that the MYB domain proteins, ASYMMETRIC LEAVES1 (AS1) from Arabidopsis thaliana,Citation1 ROUGH SHEATH2 (RS2) from maize (Zea may),Citation2,Citation3 and PHANTASTICA (PHAN) from snapdragon (Antirrhinum majus),Citation4,Citation5 and class III HOMEODOMAIN-LEUCINE ZIPPER transcription factors (HD-ZIPIII)Citation6 are required for specifying the leaf adaxial identity; whereas YABBYCitation7,Citation8 and KANADICitation6,Citation9 specify the leaf abaxial identity. On the other hand, microRNAs, miR165 and miR166, have been shown to accumulate in the leaf abaxial domain and target HD-ZIPIII genes for degradation and thereof play a role in regulating leaf adaxial-abaxial polarity establishment in A. thaliana.Citation10-Citation13
Recently, we have shown that the Medicago phantastica mutant, mtphan, in which the expression of the endogenous gene was greatly reduced by insertion of a Tnt1 retrotransposon at the 3′-end of the coding sequence, exhibits multiple leaf developmental defects.Citation14 For example, all leaf blades downwardly curled at the proximal region and developed deep serrations at the distal margin. In mature mutant plants, epidermal cells at the leaf adaxial surface were less differentiated, in contrast to the jigsaw puzzle-like leaf pavement cells of wild type plants. Furthermore, some extra tissues with distinct boundaries were formed on the leaf adaxial surface of mature mutant plants. These observations are consistent with a role of MtPHAN in the establishment and maintenance of proper leaf polarity in Medicago.Citation14
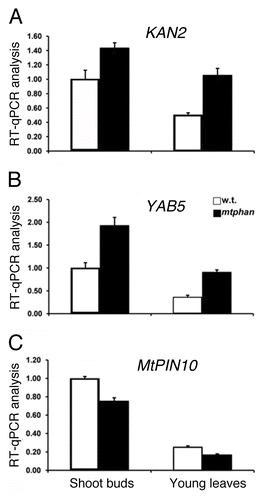
To support the phenotypic observations, we performed gene expression analysis. To do this, we first identified Medicago KANADI (KAN2) and YABBY (YAB5) genes (). Next, we compared their expression levels between wild-type and the mtphan mutant. Quantitative reverse transcription (RT)-PCR results show that KAN2 and YAB5 genes were upregulated in vegetative shoot buds and young leaves of one-month-old mtphan mutant plants compared with wild-type plants (). These results support a compromised adaxial-abaxial polarity in the mtphan mutant, as shown in other phan mutants. In compound leaf species, the sequence and location of initiation of leaflets in leaf primordia defines the final leaf pattern. In a large number of leaves of young mtphan mutant plants, lateral leaflets were developed asymmetrically on petioles, in contrast to corresponding wild-type leaves in which the pair of lateral leaflets was developed symmetrically.Citation14 This phenomenon was less pronounced in mature mutant plants. So far, it is not clear what causes this alteration. In the Medicago pin10 mutant, some lateral leaflets were also asymmetric, suggesting that local auxin activity maxima mediated by the auxin efflux transport protein are likely involved in this process.Citation14,Citation15 Consistent with this, RT-qPCR results show that the expression level of MtPIN10 is reduced in both vegetative shoot buds and young leaves of one month-old mtphan mutant compared with wild-type plants ().
Table 1. Primer sequences
Figure 1. Quantitative RT-PCR analysis of leaf polarity and patterning gene expression in Medicago truncatula. (A) KAN2, (B) YAB5, and (C) MtPIN10 gene expression was analyzed in shoot buds and young leaves of one-month-old wild-type (R108) and mtphan mutant. The expression level was normalized using a Medicago ACTIN gene. Shown are means ± s.d. (n = 3).
It has been shown that SGL1 and FCL1 are two key regulators of petiole development in Medicago truncatula.Citation16,Citation17 Mutations of each gene lead to drastically reduced petioles; whereas double mutants lack petioles at all, indicating independent function of SGL1 and FCL1 in petiole development in Medicago. In mature mtphan mutant plants that have begun reproductive phase of growth, petioles were drastically reduced; whereas rachises were greatly increased compared with wild-type counterparts.Citation14 Scanning electron microscopic observations show that petiole epidermal cells were greatly reduced in size and had extensive cell surface modifications as indicated by elaborate longitudinal and transverse folds in mature mtphan mutant, which were absent in wild-type plants. In addition, cross sections show that a single enlarged central vascular bundle was present in petioles of mature mtphan mutant. By contrast, a large vascular bundle and two small ones were present at the abaxial and adaxial sites, respectively in corresponding wild-type plants. These morphological features resemble that of the motor organ located at the end of leaflets, suggesting ectopic acquisition of the motor organ characteristics in petioles of mature mtphan mutant.Citation14
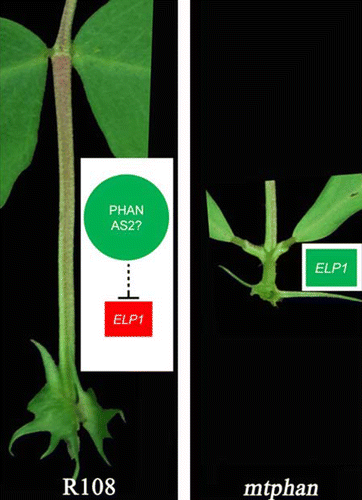
Previously, we have shown that ELONGATIED PETIOLULE1 (ELP1), which encodes a LOB domain transcription factor, determines the motor organ identity.Citation18 Ectopic overexpression of ELP1 results in petioles and rachises acquiring characteristics of the motor organ.Citation18 Because of their similarities, we hypothesized that the altered petiole phenotypes of mature mtphan mutant plants was caused by ectopic expression of ELP1. Gene expression analysis showed that ELP1 is ectopically expressed in petioles of mature mtphan mutant. In addition, the altered petiole phenotypes of mtphan were rescued to the wild-type level in mature mtphan elp1 double mutant plants. These results confirm that the petiole phenotypes of mature mtphan mutant plants were caused by ectopic expression of ELP1 in petioles.Citation14 Taken together, our results indicate that the genetic control of the petiole identity centers on suppression of ELP1 by MtPHAN in petioles (). Furthermore, we postulate that MtPHAN is involved in restricting ELP1 gene expression to the motor organ and its precursor cells. In Arabidopsis thaliana, AS1 is known to interact with the LOB domain transcription factor, AS2, to regulate expression of downstream genes, such as AUXIN RESPONSE FACTOR3 (ARF3).Citation19 It is plausible that a conserved mechanism in regulating AS1-AS2 downstream gene expression may operate in Medicago. Interestingly, the full-length MtPHAN genomic sequence including its promoter and coding sequences rescued both leaf and inflorescence phenotypes of Arabidopsis as1 mutant, suggesting that AS1 and MtPHAN are functional orthologs and furthermore the MtPHAN promoter sequence was properly recognized in Arabidopsis.Citation14
Figure 2. Models of petiole identity regulation in Medicago truncatula. MtPHAN, likely with the AS2 ortholog, represses expression of ELP1, preventing ectopic acquisition of motor organ identity in petioles of wild-type plants. In the absence of MtPHAN, ELP1 is ectopically expressed to promote motor organ identity in petioles of mature mtphan mutant. Red and green colors indicate active and inactive gene expression, respectively.
Studies in the past decades have shown that the MYB domain proteins, AS1/RS2/PHAN, play a key role in leaf polarity establishment and organ initiation. Our work confirms a role of MtPHAN in leaf polarity regulation. In addition, our work identifies a novel role of MtPHAN in petiole identity regulation in a legume species with compound leaves. These findings provide an opportunity to investigate the mode of action of MtPHAN in petiole identity regulation in future.
Materials and Methods
Plant materials and growth conditions
Medicago phan (mtphan) and wild type (R108) were used and described previously.Citation14 Plants were grown in growth chambers and glasshouses under the following conditions, 16h/8h day/night light cycle; 22 °C/20 °C day/night temperature).
RNA isolation and quantitative PCR
Shoot buds and young leaves were collected from one-month-old plants and frozen immediately in liquid nitrogen. Total RNA was isolated using RNeasy Plant Mini Kit (Qiagen) and quantified with a Nanodrop Spectrophotometer. Reverse transcription was performed using Qiagen SuperScript II Kit (Qiagen). Quantitative PCR was conducted on 7900HT Fast Real-Time PCR system (Applied Biosystems). An Medicago ACTIN gene was used to normalize the expression level. LinRegPCRCitation20 and SDS2.2.1 program (Applied Biosystems) were used for data analysis. Primer sequences are listed in .
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank members of the Chen laboratory for helpful discussions and Colleen Elles for excellent greenhouse assistance. This work was supported by The Samuel Roberts Noble Foundation.
References
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 2000; 408:967 - 71; http://dx.doi.org/10.1038/35050091; PMID: 11140682
- Timmermans MC, Hudson A, Becraft PW, Nelson T. ROUGH SHEATH2: a Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 1999; 284:151 - 3; http://dx.doi.org/10.1126/science.284.5411.151; PMID: 10102816
- Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA. The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science 1999; 284:154 - 6; http://dx.doi.org/10.1126/science.284.5411.154; PMID: 10102817
- Waites R, Hudson A. phantastica: a gene required for dorsoventrality of leaves in Antirrhinum majus.. Development 1995; 121:2143 - 54
- Waites R, Selvadurai HR, Oliver IR, Hudson A. The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum.. Cell 1998; 93:779 - 89; http://dx.doi.org/10.1016/S0092-8674(00)81439-7; PMID: 9630222
- Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol 2003; 13:1768 - 74; http://dx.doi.org/10.1016/j.cub.2003.09.035; PMID: 14561401
- Golz JF, Roccaro M, Kuzoff R, Hudson A. GRAMINIFOLIA promotes growth and polarity of Antirrhinum leaves. Development 2004; 131:3661 - 70; http://dx.doi.org/10.1242/dev.01221; PMID: 15229175
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis.. Development 1999; 126:4117 - 28; PMID: 10457020
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis.. Nature 2001; 411:706 - 9; http://dx.doi.org/10.1038/35079629; PMID: 11395775
- Williams L, Grigg SP, Xie M, Christensen S, Fletcher JC. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 2005; 132:3657 - 68; http://dx.doi.org/10.1242/dev.01942; PMID: 16033795
- Li H, Xu L, Wang H, Yuan Z, Cao X, Yang Z, Zhang D, Xu Y, Huang H. The Putative RNA-dependent RNA polymerase RDR6 acts synergistically with ASYMMETRIC LEAVES1 and 2 to repress BREVIPEDICELLUS and MicroRNA165/166 in Arabidopsis leaf development. Plant Cell 2005; 17:2157 - 71; http://dx.doi.org/10.1105/tpc.105.033449; PMID: 16006579
- Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, Barton MK, Bartel DP. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5′ region. EMBO J 2004; 23:3356 - 64; http://dx.doi.org/10.1038/sj.emboj.7600340; PMID: 15282547
- Juarez MT, Twigg RW, Timmermans MC. Specification of adaxial cell fate during maize leaf development. Development 2004; 131:4533 - 44; http://dx.doi.org/10.1242/dev.01328; PMID: 15342478
- Ge L, Peng J, Berbel A, Madueño F, Chen R. Regulation of compound leaf development by PHANTASTICA in Medicago truncatula.. Plant Physiol 2014; 164:216 - 28; http://dx.doi.org/10.1104/pp.113.229914; PMID: 24218492
- Peng J, Chen R. Auxin efflux transporter MtPIN10 regulates compound leaf and flower development in Medicago truncatula.. Plant Signal Behav 2011; 6:1537 - 44; http://dx.doi.org/10.4161/psb.6.10.17326; PMID: 21900740
- Peng J, Yu J, Wang H, Guo Y, Li G, Bai G, Chen R. Regulation of compound leaf development in Medicago truncatula by fused compound leaf1, a class M KNOX gene. Plant Cell 2011; 23:3929 - 43; http://dx.doi.org/10.1105/tpc.111.089128; PMID: 22080596
- Wang H, Chen J, Wen J, Tadege M, Li G, Liu Y, Mysore KS, Ratet P, Chen R. Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula.. Plant Physiol 2008; 146:1759 - 72; http://dx.doi.org/10.1104/pp.108.117044; PMID: 18287485
- Chen J, Moreau C, Liu Y, Kawaguchi M, Hofer J, Ellis N, Chen R. Conserved genetic determinant of motor organ identity in Medicago truncatula and related legumes. Proc Natl Acad Sci U S A 2012; 109:11723 - 8; http://dx.doi.org/10.1073/pnas.1204566109; PMID: 22689967
- Iwasaki M, Takahashi H, Iwakawa H, Nakagawa A, Ishikawa T, Tanaka H, Matsumura Y, Pekker I, Eshed Y, Vial-Pradel S, et al. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development 2013; 140:1958 - 69; http://dx.doi.org/10.1242/dev.085365; PMID: 23571218
- Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 2003; 339:62 - 6; http://dx.doi.org/10.1016/S0304-3940(02)01423-4; PMID: 12618301
