Abstract
The production of free oxylipins in plants is exquisitely controlled by cellular mechanisms that respond to environmental factors such as mechanical damage, insect herbivory and pathogen infection. One of the main targets of these cellular mechanisms are glycerolipases class A (GLA); acyl-hydrolyzing enzymes that upon their biochemical activation release unsaturated fatty acids or esterified oxylipins from glycerolipids. Recent studies performed in the wild tobacco species Nicotiana attenuata have started to unveil the complexity and specificity of GLA-regulated free oxylipin production. I present a model in which individual GLA lipases associate with individual lipoxygenases (LOX) in chloroplast membranes and envelope to define the initial committed steps of distinct oxylipin biosynthesis pathways. The unravelling of the mechanisms that activate GLAs and LOXs at the biochemical level and that control the interaction between these enzymes and their association with membranes will prove to be fundamental to understand how plants control free oxylipin biogenesis.
Recent studies describing the function of GLYCEROLIPASE 1 (GLA1) in the wild tobacco species Nicotiana attenuata have made evident that individual lipases act in coordination with individual lipoxygenases (LOXs) to define the first committed steps of distinct oxylipin biosynthesis pathways.Citation1-Citation3 By analogy with the cellular mechanisms initiating mammalian oxylipin biosynthesis,Citation4 lipases and LOXs are thought to be central targets of activating signal transduction pathways in plants.Citation5 However, in contrast to the well-characterized biochemical activation of phospholipase A2 (PLA2) and LOXs in mammalian cells,Citation4 the actual mechanisms leading to the biochemical activation of analogous oxylipin biosynthesis enzymes in plant cells remain essentially unknown.Citation5
The first lipase reported to mediate free oxylipin formation in plants was identified in the AOS (allene oxide synthase) biosynthesis pathway. The Arabidopsis thaliana DAD1 (DEFECTIVE IN ANTHER DEHISCIENCE 1) lipase was found to be essential for the developmentally controlled formation of JA in flowers.Citation6 Later, the Arabidopsis DONGLE (DGL) and AtPLAI were identified as lipases involved in the wound-induced and basal biosynthesis of JA, respectively.Citation7,Citation8 Ellinger and coworkers have revisited the participation of these and additional lipases in the supply of substrates for JA biosynthesis in Arabidopsis.Citation9 The results of the study indicated that the wound- and pathogen-mediated accumulation of JA in Arabidopsis is determined by multiple lipases acting at different stages of the wound and infection response. Consistent with this conclusion, it has been recently reported that the transcriptional control of Arabidopsis DAD1-like lipases (DLL) is under regulation of a complex signaling network.Citation10 As certain oxylipins can accumulate or be formed directly in glycerolipids (i.e. lipid-bound oxylipins),Citation11,Citation12 the lipase-catalyzed release of these esterified oxylipins into the free oxylipin pool adds an additional level of complexity to the regulation and dynamics of free oxylipin accumulation.Citation9,Citation13
Similar to DAD1, DGL and AtPLAI, N. attenuata GLA1 belongs to the phospholipase-A1 class I (PLA1-I) family of phospholipases which is characterized by the presence of an N-terminal chloroplast transit peptide and a conserved lipase-3 domain.Citation14 By definition, phospholipases class A (PLA) de-esterify acyl groups from phospholipids, but several lipases involved in JA formation have been shown to also cleave in vitro acyl groups from galactolipids and triacylglycerols.Citation1,Citation6 Thus, we have previously adopted the more general name glycerolipase A (GLA) to refer to lipases class A involved in the biogenesis of free oxylipins in plants.Citation1-Citation3
GLA1 supplies substrates to the AOS pathway upon wounding and herbivory however it does not supply substrates to this pathway during flower development.Citation2 Moreover, GLA1 does not supply substrates to the HPL (hydroperoxide lyase) pathway upon wounding and herbivory.Citation2 Strikingly, GLA1 is essential for the supply of substrates to the cytosolic 9-LOX pathway and the formation of hydrolxylated fatty acids and other C18 and C19 derivatives during P. parasitica infection.Citation3 Yet, GLA1 does not supply the DES (divinyl ether synthase) pathway for DVE (divinyl ether) formation during infection by this pathogen.Citation2 Together, these results clearly indicate that individual 9- and 13-LOXs associate with individual GLAs to control the entry points of plastidial and cytosolic free oxylipin biosynthesis pathways. Importantly, since GLA1 localizes to the plastid (to which sub-organellar compartment in particular has not yet been defined),Citation1 the results also suggest that 9-LOXs associate with GLA1 in the chloroplast intermembrane space or envelope. This conclusion is consistent with the capacity of GLA1 to access phosphatidylcholine (PC) –a constituent of the chloroplast envelope– in vivo.Citation2 Based on this GLA1/9-LOX metabolic association, the model presented in puts forward the concept that DVE formation is also initiated in the chloroplast membrane environment (placed in the envelope in but considering that the intermembrane space is also a plausible location for GLA1). In summary, the model presented in proposes that the chloroplast -as the centerpiece of oxylipin formation in plants- hosts the entry points of all GLA/LOX-dependent free oxylipin biosynthesis pathways.
Figure 1. GLA-mediated biosynthesis of free oxylipins in leaves after wounding, chewing insect hervibory and P. parasitica infection. After tissue damage by abiotic factors or herbivory, and after tissue infection by pathogens (e.g., P. parasitica), cellular signal transduction pathways trigger the biochemical activation of pathway-specific GLA lipases, residing (otherwise inactive) in the membrane environments of the chloroplast (see text for more details). Some plant species have the capacity to accumulate substantial amounts of oxylipins esterified to membrane or non-membrane glycerolipids, and the model includes the putative pathways (labeled with a question mark) in which GLA lipases may participate in the release of esterified oxylipins from membrane glycerolipids. 13-LOX derived oxylipins can be metabolized inside or outside chloroplasts (e.g., OPDA, C6 aldehydes, C12 oxo-acids) and exported from this organelle or released outside cells (e.g., C6 volatiles, methyl-JA). *In N. attenuata, LOX2 supplies substrates specifically to the HPL pathway while LOX3 to the AOS pathway after mechanical damage or chewing insect herbivory. **In N. attenuata, 9-hxdroxy-C12 is mainly formed via HPL recycling activity.Citation19 Glycerolipase A (GLA), Lipoxygenase (LOX); Allene Oxide Synthase (AOS), Allene Oxide Cyclase (AOC), Hydroperoxide Lyase (HPL), Divinyl Ether Synthase (DES); JA: jasmonic acid, DVE: divinyl-ether; OPDA: 12-oxophytodienoic acid; 18:2: linoleic acid; 18:3: α-linolenic acid; OOH-FA: hydroperoxy fatty acid.
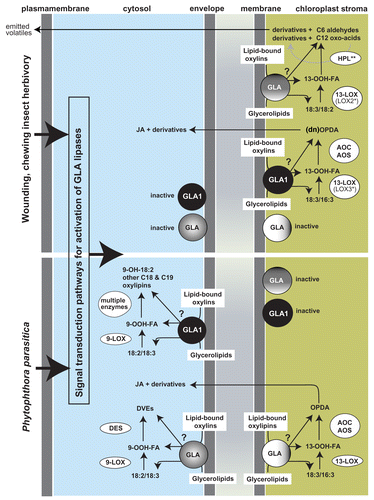
GLA1 and similar lipases are predicted to be soluble (G. Bonaventure, unpublished data), so they may associate with membranes peripherally or gain access to them upon biochemical activation. Activation mechanisms may also involve recruitment of LOXs onto membranes to define specific GLA/LOX entry points. This hypothesis is consistent with a rapid flux of free fatty acids into LOX3 upon wounding and simulated insect herbivory in N. attenuata.Citation1,Citation5 As mentioned above, the regulation of oxylipin biosynthesis in mammals is well defined, and involves processes in which calcium and phosphorylation by mitogen activated protein kinases (MAPKs) play key roles in the translocation of lipases, LOXs and scaffold proteins onto membranes.Citation4 In Arabidopsis, calcium signaling may play an important role in the activation of LOXs for oxylipin biosynthesis,Citation15 and in tobacco and Arabidopsis, MAPK signaling is essential for the activation of oxylipin biosynthesis.Citation1,Citation16-Citation18 As mentioned above, the unravelling of the mechanisms that activate GLAs and LOXs at the biochemical level and that control the interaction between these enzymes and their association with membranes will prove to be fundamental to understand how plants so exquisitely control free oxylipin biogenesis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kallenbach M, Alagna F, Baldwin IT, Bonaventure G. Nicotiana attenuata SIPK, WIPK, NPR1, and fatty acid-amino acid conjugates participate in the induction of jasmonic acid biosynthesis by affecting early enzymatic steps in the pathway. Plant Physiol 2010; 152:96 - 106; http://dx.doi.org/10.1104/pp.109.149013; PMID: 19897603
- Bonaventure G, Schuck S, Baldwin IT. Revealing complexity and specificity in the activation of lipase-mediated oxylipin biosynthesis: a specific role of the Nicotiana attenuata GLA1 lipase in the activation of jasmonic acid biosynthesis in leaves and roots. Plant Cell Environ 2011; 34:1507 - 20; http://dx.doi.org/10.1111/j.1365-3040.2011.02348.x; PMID: 21554327
- Schuck S, Kallenbach M, Baldwin IT, Bonaventure G. The Nicotiana attenuata GLA1 lipase controls the accumulation of Phytophthora parasitica-induced oxylipins and defensive secondary metabolites. Plant Cell Environ 2014; forthcoming http://dx.doi.org/10.1111/pce.12281; PMID: 24450863
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 2001; 294:1871 - 5; http://dx.doi.org/10.1126/science.294.5548.1871; PMID: 11729303
- Bonaventure G, Baldwin IT. New insights into the early biochemical activation of jasmonic acid biosynthesis in leaves. Plant Signal Behav 2010; 5:287 - 9; http://dx.doi.org/10.4161/psb.5.3.10713; PMID: 20037473
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 2001; 13:2191 - 209; PMID: 11595796
- Hyun Y, Choi S, Hwang HJ, Yu J, Nam SJ, Ko J, Park JY, Seo YS, Kim EY, Ryu SB, et al. Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev Cell 2008; 14:183 - 92; http://dx.doi.org/10.1016/j.devcel.2007.11.010; PMID: 18267087
- Yang W, Devaiah SP, Pan X, Isaac G, Welti R, Wang X. AtPLAI is an acyl hydrolase involved in basal jasmonic acid production and Arabidopsis resistance to Botrytis cinerea. J Biol Chem 2007; 282:18116 - 28; http://dx.doi.org/10.1074/jbc.M700405200; PMID: 17475618
- Ellinger D, Stingl N, Kubigsteltig II, Bals T, Juenger M, Pollmann S, Berger S, Schuenemann D, Mueller MJ. DONGLE and DEFECTIVE IN ANTHER DEHISCENCE1 lipases are not essential for wound- and pathogen-induced jasmonate biosynthesis: redundant lipases contribute to jasmonate formation. Plant Physiol 2010; 153:114 - 27; http://dx.doi.org/10.1104/pp.110.155093; PMID: 20348210
- Ruduś I, Terai H, Shimizu T, Kojima H, Hattori K, Nishimori Y, Tsukagoshi H, Kamiya Y, Seo M, Nakamura K, et al. Wound-induced expression of DEFECTIVE IN ANTHER DEHISCENCE1 and DAD1-like lipase genes is mediated by both CORONATINE INSENSITIVE1-dependent and independent pathways in Arabidopsis thaliana.. Plant Cell Rep 2014; forthcoming PMID: 24430866
- Stelmach BA, Müller A, Hennig P, Gebhardt S, Schubert-Zsilavecz M, Weiler EW. A novel class of oxylipins, sn1-O-(12-oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl Diglyceride, from Arabidopsis thaliana.. J Biol Chem 2001; 276:12832 - 8; http://dx.doi.org/10.1074/jbc.M010743200; PMID: 11278736
- Böttcher C, Weiler EW. cyclo-Oxylipin-galactolipids in plants: occurrence and dynamics. Planta 2007; 226:629 - 37; http://dx.doi.org/10.1007/s00425-007-0511-5; PMID: 17404756
- Nakashima A, von Reuss SH, Tasaka H, Nomura M, Mochizuki S, Iijima Y, Aoki K, Shibata D, Boland W, Takabayashi J, et al. Traumatin- and dinortraumatin-containing galactolipids in Arabidopsis: their formation in tissue-disrupted leaves as counterparts of green leaf volatiles. J Biol Chem 2013; 288:26078 - 88; http://dx.doi.org/10.1074/jbc.M113.487959; PMID: 23888054
- Ryu SB. Phospholipid-derived signaling mediated by phospholipase A in plants. Trends Plant Sci 2004; 9:229 - 35; http://dx.doi.org/10.1016/j.tplants.2004.03.004; PMID: 15130548
- Bonaventure G, Gfeller A, Proebsting WM, Hörtensteiner S, Chételat A, Martinoia E, Farmer EE. A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J 2007; 49:889 - 98; http://dx.doi.org/10.1111/j.1365-313X.2006.03002.x; PMID: 17253984
- Seo S, Sano H, Ohashi Y. Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 1999; 11:289 - 98; PMID: 9927645
- Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 2007; 19:805 - 18; http://dx.doi.org/10.1105/tpc.106.046581; PMID: 17369371
- Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata.. Plant Cell 2007; 19:1096 - 122; http://dx.doi.org/10.1105/tpc.106.049353; PMID: 17400894
- Kallenbach M, Gilardoni PA, Allmann S, Baldwin IT, Bonaventure G. C12 derivatives of the hydroperoxide lyase pathway are produced by product recycling through lipoxygenase-2 in Nicotiana attenuata leaves. New Phytol 2011; 191:1054 - 68; http://dx.doi.org/10.1111/j.1469-8137.2011.03767.x; PMID: 21615741
