Abstract
Heterotrimeric G-proteins (G-proteins, hereafter) are important signaling components in all eukaryotes. The absence of these proteins in the sequenced genomes of Chlorophycean green algae has raised questions about their evolutionary origin and prevalence in the plant lineage. The existence of G-proteins has often been correlated with the acquisition of embryophytic life-cycle and/or terrestrial habitats of plants which occurred around 450 million years ago. Our discovery of functional G-proteins in Chara braunii, a representative of the Charophycean green algae, establishes the existence of this conserved signaling pathway in the most basal plants and dates it even further back to 1–1.5 billion years ago. We have now identified the sequence homologs of G-proteins in additional algal families and propose that green algae represent a model system for one of the most basal forms of G-protein signaling known to exist to date. Given the possible differences that exist between plant and metazoan G-protein signaling mechanisms, such basal organisms will serve as important resources to trace the evolutionary origin of proposed mechanistic differences between the systems as well as their plant-specific functions.
Introduction
Heterotrimeric G-proteins are key signaling molecules involved in regulation of a wide spectrum of signal transduction pathways established in different eukaryotic organisms.Citation1 The basic molecular mechanism entails a switch-like signaling process with an inactive state comprised of a trimer consisting of the three subunits Gα, Gβ and Gγ, and an active state characterized by the dissociation of Gα subunit from the Gβ/Gγ dimer. The interchange between both these states is dependent on the exchange of GDP against GTP for activation and the subsequent GTP hydrolysis for inactivation on the Gα subunit.Citation2,Citation3 The G-protein signaling mechanism in plants is not yet understood in the exquisite details that are available for metazoan systems where activation is exclusively mediated by the guanine nucleotide exchange (GEF) activity of a cognate G-protein coupled receptor (GPCR). In metazoans, it has been established that ligand binding to a GPCR leads to the conformational change in Gα which allows for GDP to GTP exchange. Canonical GPCRs do not seem to exist in plants and Arabidopsis Gα has been proposed to be self-activated.Citation4 The origin of such differences and its prevalence across species remains to be ascertained. Additionally, almost every heterotrophic species exhibits a lineage-specific expansion of G-proteins and their regulators, and multiple genes exist for each of the G-protein subunits. In contrast, plants have a relatively simple repertoire of G-protein genes which ranges from multiple copies in plants that have gone through recent genome duplication events, e.g., soybean (4 Gα, 4 Gβ and 10 Gγ)Citation5 to the bryophyte Physcomitrella patens (moss) that contains Gβ and Gγ subunits, but no Gα.Citation4 The most commonly-explored plants such as Arabidopsis and rice encode for one Gα, one Gβ and a few Gγ proteins.Citation6 Despite their limited quantities, plant G-proteins have been shown to be involved in regulation of multiple growth and developmental pathways, especially in modulating plant hormone responses.Citation7-Citation9
We have recently discovered that basal, aquatic plants such as the green alga Chara braunii possess each of the G-protein subunits as well as its regulatory protein, RGS (Regulator of G-protein Signaling). The basic biochemical properties of Chara G-protein genes are similar to what has been reported for land plants and the Gα and RGS proteins from Chara and Arabidopsis exhibit cross-species biochemical functionality.Citation10 We now report that G-proteins exist in additional charophyte species besides Chara braunii and their absence from the sequenced genomes of chlorophyte algae possibly reflects their loss in one specific branch of the algal lineage. These findings reaffirm that the existence of G-protein signaling in plants is not correlated with the acquisition of terrestrial habitat or predominance of embryophytic life-cycle.
Material and Methods
In silico-EST data analysis
To optimize the search for potential candidate genes and to account for different codon usage bias in different species, we used amino acid sequence query with the TBLASTNCitation11 algorithm. This allowed for screening of all six possible reading frames of target nucleotide sequences. Arabidopsis GPA1 (Accession: AEC07820), AGB1 (accession: AEE86382), AGG1 (accession: AEE80480), AGG2 (accession: AEE76694) and AGG3 (accession: Q6AWT8) were used as query for TBLASTN search using GenBank+EMBL+DDBJ database EST sequences and the nucleotide collection hosted by NCBI.
Phytohormone detection
Chara braunii were cultivated in distilled water on a sand/soil/peat mixture at room temperature and 14/10 h light/dark cycle as described previously.Citation12 Aquatic tissue was harvested and lyophilized for 16 h and subsequently ground in liquid N2. Fifty mg algal dry mass was used for acidic hormone quantification. Salicylic acid (SA), abscisic acid (ABA), jasmonic acid (JA), indole 3 acetic acid (IAA), jasmonate-isoleucine conjugate (JA-Ile), indole-3-acetyl-aspartate (IAA-Asp) and JA precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA) were assayed using an LC-MS/MS method. Hormone extraction was performed in the presence of a mixture of deuterium labeled standards (D5SA, D6ABA, D2JA, D5IAA) at 2.5 μM each which was spiked at the beginning of the extraction. Samples were homogenized twice in 900 µL MeOH/ACN (1:1 v/v) and one time in 200 μL of 30% methanol followed by analysis using Shimadzu LC system interfaced with an AB Sciex 4000 QTRAP mass spectrometer and TurboIonSpray (TIS) electrospray ion source. For LC separation, a monolithic C18 column (Onyx, 4.6 mm x 100 mm, Phenomenex) with a guard cartridge was used flowing at 1 ml.min–1. The gradient was from 60% solvent A (0.1% [v/v] acetic acid in Milli-Q water) to 100% solvent B (90% acetonitrile [v/v] with 0.1% acetic acid [v/v]). Hormones were detected in three technical replicates using MRM transitions and quantified with standard samples.
Results and Discussion
The absence of G-protein genes in chlorophytes and their presence in Chara braunii prompted us to uncover their distribution in additional algal genomes. Our analysis shows that genes encoding G-protein subunits exist in multiple charophyte algae. In general, green algae can be divided in two major divisions: Chlorophyta and Charophyta, which represent two polyphyletic sister branches.Citation13-Citation15 Charophyta, together with land plants form the monophyletic branch of Streptophyta and comprise six different taxa (Fig. 0.1): Mesostigmatophyceae, Chlorokybophyceae, Klebsormidiophyceae, Zygnematophyceae, Coleochaetophyceae and Charophyceae.Citation16-Citation18 Interestingly, both monotypic taxa, Mesostigmatophyceae and Chlorokybophyceae, with Mesostigma viride and Chlorokybus atmophyticus as their only known representatives, do not show the presence of candidate genes encoding G-protein subunits in our ESTs data analysis. Mesostigma is unicellular and its phylogenetic rank under the green algae is still controversial. It is also unclear if it belongs to the plant lineage or represents a unique sister to all green algae.Citation19-Citation22 Conversely, Chlorokybus belongs to the charophyte green algae and forms conglomerates of several cells possessing a non-motile vegetative phase, an evolutionary innovation which seems to be absent in Mesostigma.Citation17
The earliest charophyte species possessing ESTs with a high similarity to the Arabidopsis thaliana G-proteins identified in our studies was Klebsormidium flaccidum (Klebsormidiophyceae), which contains orthologous sequences to AtGPA1 and AtAGB1. In addition, Coleochaete scutata, Chaetosphaeridium globosum (Coleochaetophyceae) and Nitella hyalina (Charophyceae) also exhibit one orthologous gene for the Gα subunit (). No promising candidates for G-protein genes could be identified in Zygnematophyceae so far. Whole genome sequencing data, which are not available for any of the charophyte green alga to date, will certainly help complete the picture of heterotrimeric G-proteins in green algae and therefore also in land plant evolution. This could be particularly true for Zygnematophyceae where G-protein genes seem to be absent but are expected to exist due to their phylogeny as sister to Coleochaetophyceae and/or Charophyceae. Zygnematophyceae are considered to be more advanced than Klebsormidiophyceae in the charophyte lineageCitation16 and exhibit enormous diversity, with more than 4000 species and morphologies which range from unicellular to filamentous.Citation17,Citation20
Figure 1. Schematic overview of the phylogeny of green algae and evolutionary innovations (modified from).Citation16,Citation20 Potential genes encoding Gα, Gβ and Gγ subunits of the heterotrimeric G protein complex based on EST data analysis are labeled with orange, blue or green and selected morphological or molecular characters are shown. G-protein genes can be found in the monophyletic branch of Streptophyta such as in several charophytes (Klebsormidiophyceae, Coleochaetophyceae and Charophyceae) and embryophytes, but not in chlorophytes.
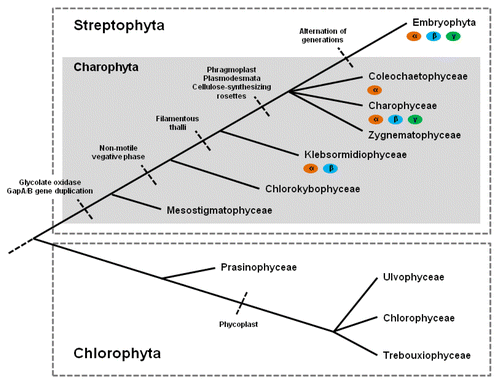
No representatives of the chlorophyte algae showed evidence for the presence of heterotrimeric G protein genes so far, which is especially remarkable because the genomes of several chlorophytes (Volvox carteri, Chlamydomonas reinhardtii, Cocomyxa subellipsoidea C-169, Micromonas pusilla CCMP1545, Micromonas pusilla RCC299 and Ostreococcus lucimarinus) are completely sequenced.Citation23 In fact, no G-protein homologs were identified among the glaucophytes or rodophytes (algal lineages more primitive than chlorophytes), suggesting Klebsormidiophyceae are the most primitive green plants that contain G-proteins. In general, due to the limited number of fully sequenced genomes, these findings should be considered with care. However, it is worth mentioning that the limiting factor for the identification of G-protein genes is the completeness of transcriptome datasets rather than the sequence homology of the conserved protein domains in the search. Sequence-based homology searches have successfully identified G-protein genes in a variety of plants.Citation24 Nonetheless, it can be stated without doubt that G-protein signaling of photo-autotrophic organisms evolved early in the plant lineage, long before embryophytes conquered terrestrial habitats. Furthermore, the origin of heterotrimeric G-protein signaling can be dated before major innovations like the appearance of phragmoplasts, but after or concurrent with the appearance of multicellular, filamentous morphology. This is based on the fact that Klebsormidium flaccidum, which contains G-protein genes, is a multicellular, non-branching green alga whose ancestor separated before the innovation of phragmoplasts, plasmodesmata, branching, apical cell growth, desiccation-resistant zygospores or cellulose-synthesizing rosettes.Citation16,Citation17
Besides charophyte green algae, we identified homologs of Arabidopsis Gα and Gβ proteins in the sequenced genomes of two other basal photosynthetic organisms, the diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum.Citation25,Citation26 Diatoms represent mostly unicellular algae and are commonly described as part of phytoplankton. Diatoms are not monophyletic with green algae and belong instead to the “supergroup” of Chromalveolates which include a plethora of different algae possessing plastids derived from a red algal endosymbiont.Citation16 A large number of green algal genes can be found in Chromalveolates genomesCitation27 and although its phylogeny is not yet completely understood, it is supposed that these genes were incorporated in the Chromalveolates genomes by endosymbiosis of green algal ancestors.Citation28 These data further corroborate that G-protein genes have been lost only in the specific green algae lineages.
Since we are only beginning to uncover the existence of heterotrimeric G-proteins in basal plant species, it is expected that huge knowledge gaps exist concerning their evolution and function in these lineages. One of the major roles of higher plant G-proteins comprises the regulation of growth, development and stress responses by modulation of plant hormone signal transduction pathways. As a first step to evaluate the possible roles of G-proteins in green algae, we elucidated the presence of plant hormones in Chara braunii. Our data show the unambiguous presence of SA, ABA and IAA, whereas JA as well as JA derivatives and precursors were undetectable (). Since charophytes possess multi-cellular morphology including differentiation of certain cell types, cell-to-cell communication and signaling might be necessary and G-proteins could potentially be involved in regulation of phytohormone-regulated pathways in green algae, similar to higher plants. Recent reports demonstrate the role of plant hormone-regulated processes in green algae, for example polar auxin transport in Chara corallinaCitation29 and gibberellic acid and brassinosteroid responses in Klebsormidium flaccidum.Citation30 Heterotrimeric G-proteins might also be involved in some of these processes since charophytes and land plants share several physiological characteristics which are absent in chlorophytes such as globular cellulose synthesizing terminal complexes,Citation31 the GapA/B gene duplicationCitation32 and specific enzymes like glycolate oxidaseCitation33 or class I aldolases.Citation34 Further analysis of additional genome sequences and characterization of G-proteins in regulating key signaling pathways in algae will shed light on their evolution, expanse, function and possible divergence from established regulatory mechanisms.
Table 1. Quantification of acidic phytohormones in Chara braunii using LC-MS/MS
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by National Science Foundation grant (MCB-1157944) to SP. We are thankful to Dr. Ursula Goodenough, Biology Department, Washington University, St. Louis and Dr. Jae-Hyeok Lee, Department of Botany, University of British Columbia for insightful discussions.
References
- Bradford W, Buckholz A, Morton J, Price C, Jones AM, Urano D. Eukaryotic G protein signaling evolved to require G protein-coupled receptors for activation. Sci Signal 2013; 6:ra37; http://dx.doi.org/10.1126/scisignal.2003768; PMID: 23695163
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev 2003; 24:765 - 81; http://dx.doi.org/10.1210/er.2000-0026; PMID: 14671004
- Offermanns S. G-proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol 2003; 83:101 - 30; http://dx.doi.org/10.1016/S0079-6107(03)00052-X; PMID: 12865075
- Urano D, Jones JC, Wang H, Matthews M, Bradford W, Bennetzen JL, Jones AM. G protein activation without a GEF in the plant kingdom. PLoS Genet 2012; 8:e1002756; http://dx.doi.org/10.1371/journal.pgen.1002756; PMID: 22761582
- Choudhury SR, Westfall CS, Pandey S. The RGS proteins add to the diversity of soybean heterotrimeric G-protein signaling. Plant Signal Behav 2012; 7:1114 - 7; http://dx.doi.org/10.4161/psb.21298; PMID: 22899066
- Urano D, Chen JG, Botella JR, Jones AM. Heterotrimeric G protein signalling in the plant kingdom. Open Biol 2013; 3:120186; http://dx.doi.org/10.1098/rsob.120186; PMID: 23536550
- Li S, Liu Y, Zheng L, Chen L, Li N, Corke F, Lu Y, Fu X, Zhu Z, Bevan MW, et al. The plant-specific G protein γ subunit AGG3 influences organ size and shape in Arabidopsis thaliana. New Phytol 2012; 194:690 - 703; http://dx.doi.org/10.1111/j.1469-8137.2012.04083.x; PMID: 22380792
- Tsugama D, Liu S, Takano T. Arabidopsis heterotrimeric G protein β subunit, AGB1, regulates brassinosteroid signalling independently of BZR1. J Exp Bot 2013; 64:3213 - 23; http://dx.doi.org/10.1093/jxb/ert159; PMID: 23814276
- Pandey S, Chen JG, Jones AM, Assmann SM. G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol 2006; 141:243 - 56; http://dx.doi.org/10.1104/pp.106.079038; PMID: 16581874
- Hackenberg D, Sakayama H, Nishiyama T, Pandey S. Characterization of the heterotrimeric G-protein complex and its regulator from the green alga Chara braunii expands the evolutionary breadth of plant G-protein signaling. Plant Physiol 2013; 163:1510 - 7; http://dx.doi.org/10.1104/pp.113.230425; PMID: 24179134
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403 - 10; PMID: 2231712
- Schmölzer PM, Höftberger M, Foissner I. Plasma membrane domains participate in pH banding of Chara internodal cells. Plant Cell Physiol 2011; 52:1274 - 88; http://dx.doi.org/10.1093/pcp/pcr074; PMID: 21659328
- Lemieux C, Otis C, Turmel M. A clade uniting the green algae Mesostigma viride and Chlorokybus atmophyticus represents the deepest branch of the Streptophyta in chloroplast genome-based phylogenies. BMC Biol 2007; 5:2; http://dx.doi.org/10.1186/1741-7007-5-2; PMID: 17222354
- Bremer K. Summary of green plant phylogeny and classification. Cladistics 1985; 1:369 - 85; http://dx.doi.org/10.1111/j.1096-0031.1985.tb00434.x
- Kraepelin G. Green Algae. Structure, Reproduction and Evolution in Selected Genera. Z Allg Mikrobiol 1977; 17:170; http://dx.doi.org/10.1002/jobm.3630170220
- Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF, De Clerck O. Phylogeny and Molecular Evolution of the Green Algae. Crit Rev Plant Sci 2012; 31:1 - 46; http://dx.doi.org/10.1080/07352689.2011.615705
- McCourt RM, Delwiche CF, Karol KG. Charophyte algae and land plant origins. Trends Ecol Evol 2004; 19:661 - 6; http://dx.doi.org/10.1016/j.tree.2004.09.013; PMID: 16701329
- Qiu YL, Palmer JD. Phylogeny of early land plants: insights from genes and genomes. Trends Plant Sci 1999; 4:26 - 30; http://dx.doi.org/10.1016/S1360-1385(98)01361-2; PMID: 10234267
- Bhattacharya D, Weber K, An SS, Berning-Koch W. Actin phylogeny identifies Mesostigma viride as a flagellate ancestor of the land plants. J Mol Evol 1998; 47:544 - 50; http://dx.doi.org/10.1007/PL00006410; PMID: 9797404
- Delwiche CF, Karol KG, Cimino MT, Sytsma KJ. Phylogeny of the genus Coleochaete (Coleochaetales, charohyta) and related taxa inferred by analysis of the chloroplast gene rbcL1. J Phycol 2002; 38:394 - 403; http://dx.doi.org/10.1046/j.1529-8817.2002.01174.x
- Turmel M, Otis C, Lemieux C. The complete mitochondrial DNA sequence of Mesostigma viride identifies this green alga as the earliest green plant divergence and predicts a highly compact mitochondrial genome in the ancestor of all green plants. Mol Biol Evol 2002; 19:24 - 38; http://dx.doi.org/10.1093/oxfordjournals.molbev.a003979; PMID: 11752187
- Lemieux C, Otis C, Turmel M. Ancestral chloroplast genome in Mesostigma viride reveals an early branch of green plant evolution. Nature 2000; 403:649 - 52; http://dx.doi.org/10.1038/35001059; PMID: 10688199
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 2012; 40:D1178 - 86; http://dx.doi.org/10.1093/nar/gkr944; PMID: 22110026
- Trusov Y, Chakravorty D, Botella JR. Diversity of heterotrimeric G-protein γ subunits in plants. BMC Res Notes 2012; 5:608; http://dx.doi.org/10.1186/1756-0500-5-608; PMID: 23113884
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, et al. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 2004; 306:79 - 86; http://dx.doi.org/10.1126/science.1101156; PMID: 15459382
- Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008; 456:239 - 44; http://dx.doi.org/10.1038/nature07410; PMID: 18923393
- Moustafa A, Beszteri B, Maier UG, Bowler C, Valentin K, Bhattacharya D. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 2009; 324:1724 - 6; http://dx.doi.org/10.1126/science.1172983; PMID: 19556510
- Dorrell RG, Smith AG. Do red and green make brown?: perspectives on plastid acquisitions within chromalveolates. Eukaryot Cell 2011; 10:856 - 68; http://dx.doi.org/10.1128/EC.00326-10; PMID: 21622904
- Boot KJ, Libbenga KR, Hille SC, Offringa R, van Duijn B. Polar auxin transport: an early invention. J Exp Bot 2012; 63:4213 - 8; http://dx.doi.org/10.1093/jxb/ers106; PMID: 22473986
- Stirk WA, Bálint P, Tarkowská D, Novák O, Strnad M, Ördög V, van Staden J. Hormone profiles in microalgae: gibberellins and brassinosteroids. Plant Physiol Biochem 2013; 70:348 - 53; http://dx.doi.org/10.1016/j.plaphy.2013.05.037; PMID: 23811778
- Hotchkiss AT, Brown RM. The association of rosette and globule terminal complexes with cellulose microfibril assembly in Nitella translucens var. axillaris (Charophyceae). J Phycol 1987; 23:229 - 37; http://dx.doi.org/10.1111/j.1529-8817.1987.tb04130.x
- Petersen J, Teich R, Becker B, Cerff R, Brinkmann H. The GapA/B gene duplication marks the origin of Streptophyta (charophytes and land plants). Mol Biol Evol 2006; 23:1109 - 18; http://dx.doi.org/10.1093/molbev/msj123; PMID: 16527864
- Mattox KR, Stewart KD. Classification of the green algae: a concept based on comparative cytology. In The Systematics of Green Algae, Irvine, D. E. G.; John, D. M., Eds. Academic Press: 1984; pp 29–72.
- Jacobshagen S, Schnarrenberger C. Two class I aldolases in Klebsormidium flaccidum (Charophyceae): an evolutionary link from chlorophytes to higher plants. J Phycol 1990; 26:312 - 7; http://dx.doi.org/10.1111/j.0022-3646.1990.00312.x
