?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The emission of radio-frequency electromagnetic radiation (EMR) by various wireless communication base stations has increased in recent years. While there is wide concern about the effects of EMR on humans and animals, the influence of EMR on plants is not well understood. In this study, we investigated the effect of EMR on the growth dynamics of Myriophyllum aquaticum (Parrot feather) by measuring the nanometric elongation rate fluctuation (NERF) using a statistical interferometry technique. Plants were exposed to 2 GHz EMR at a maximum of 1.42 Wm−2 for 1 h. After continuous exposure to EMR, M. aquaticum plants exhibited a statistically significant 51 ± 16% reduction in NERF standard deviation. Temperature observations revealed that EMR exposure did not cause dielectric heating of the plants. Therefore, the reduced NERF was due to a non-thermal effect caused by EMR exposure. The alteration in NERF continued for at least 2.5 h after EMR exposure and no significant recovery was found in post-EMR NERF during the experimental period.
Introduction
Various gas, liquid, and solid contaminants are known to pollute the environment. In many cases, these pollutants can accumulate in the environment and cause considerable damage before their effects are identified and control measures are undertaken (e.g., cadmium,Citation1 arsenic,Citation2 O3,Citation3 and CO2). It is therefore of great importance to study the possible impacts of potential contaminants that are not yet considered to be pollutants.
The utilization of radio-frequency electromagnetic radiation (EMR) is increasing with the extensive deployment of mobile technology base stations, particularly mobile phone and wireless broadband access base stations. Furthermore, in some locations, there can be 4 or more mobile phone service providers covering a single geological area,Citation4 leading to increased EMR densities in those environments. EMR exposure guidelines are based on the heating effect of EMR on biological tissues,Citation5 and effects of EMR on humans and other animals are areas of concern. To date, the effect of EMR on plants has not been considered to be problematic. This may be due to the assumption that environmental exposure does not have a thermal effect on plants, and that plants are less sensitive to environmental stressors than are animals. A few studies discussing EMR effects on plants have been published, but the data are insufficient to allow general conclusions to be reached. Nevertheless, some evidence suggests that plants can experience non-thermal stress as a result of exposure to EMR.Citation6-Citation11
Plants alter their growth and development when exposed to various environmental stressors and stimuli, such as toxic gases (e.g., O3 and SO2),Citation12,Citation13 magnetic fields,Citation14,Citation15 electric fields,Citation16 and vibrations.Citation17 EMR exposure may also act as an environmental stress or stimulus for plants. For example, reactive oxygen species formation increases in plant tissues after exposure to EMR.Citation6,Citation10 This has the potential to increase plant oxidative stress, which can result in a decreased growth rate.Citation18,Citation19 Investigation of the growth dynamics of plants after EMR exposure would provide a good indication of the effects of EMR on plants. As yet, however, no studies have been performed examining the effect of EMR on plant growth dynamics during or immediately after exposure. This may be due to the difficulties encountered when attempting to quantify plant growth parameters over short time periods using non-invasive techniques. To overcome these limitations, we used statistical interferometry techniques (SIT) to measure the growth dynamics of Myriophyllum aquaticum (Parrot feather) plants before and after exposure to EMR. Using this technique, we measured changes in the standard deviation of nanometric fluctuation in the M. aquaticum stem length. In stable conditions, this fluctuation remains largely unchanged for several hours. The nanometric fluctuations of the lengths of some plant parts (leaves and roots) change in response to environmental stresses, and these nanometer-scale changes can be monitored using SIT.Citation20,Citation21 To investigate any thermal effects, which can be caused by dielectric heating due to EMR exposure,Citation22 the temperature of plants before and during EMR exposure was observed using thermographic images.
Results
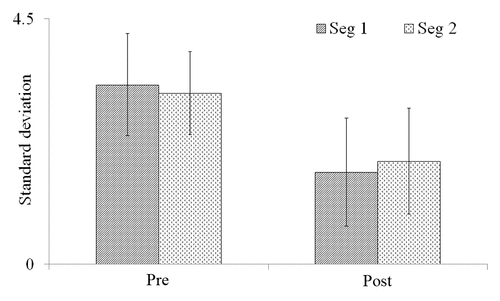
M. aquaticum stems exposed to EMR had significantly smaller standard deviations (SD) of nanometric elongation rate fluctuations (NERF) after exposure than prior to exposure (1.51 ± 0.43 vs. 3.24 ± 0.96 nm s−1 mm−1, P < 0.05). This represents a reduction in SD of 51 ± 16% from the pre-EMR exposure. By contrast, the average difference between control-plant NERF SDs before and after placement in the EMR exposure chamber was 11 ± 7%, which was not statistically significant (1.89 ± 0.89 vs. 1.71 ± 0.86 nm s−1 mm−1, P > 0.05; ). To determine whether NERF recovered toward the pre-EMR exposure state, the growth rate data for both pre- and post-EMR exposure measurements were divided into 2 30-min segments and the SD of each segment was calculated separately. The NERF SDs for the 2 pre-EMR 30-min exposure measurement periods were not significantly different from one another (3.29 ± 0.93 and 3.14 ± 0.99 nm s−1 mm−1, P > 0.05). Similarly, the NERF SDs for the 2 post-EMR exposure measurement periods were likewise not significantly different from one another (1.68 ± 0.76 and 1.89 ± 0.97 nm s−1 mm−1, P > 0.05; ).
Figure 1. Relative changes in the standard deviation of elongation rate fluctuation in M. aquaticum stems. Plants were either exposed to 2-GHz radio-frequency electromagnetic radiation (EMR; 2 GHz) or ambient-only EMR (control). Pre and Post represent pre- and post- EMR exposure, respectively. Error bars represent standard deviations.
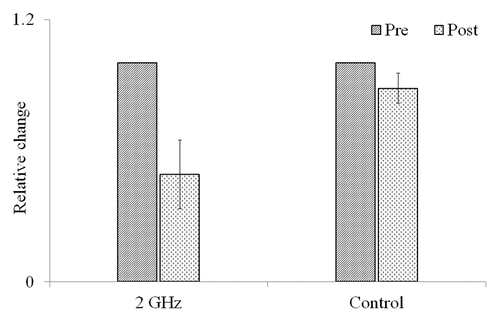
Figure 2. Standard deviation of 2 consecutive 30-min measurements of length fluctuation in M. aquaticum stems before and after exposure to 2-GHz radio-frequency electromagnetic radiation (EMR). “Seg 1” and “Seg 2” represent 2 consecutive 30-min time segments. “Pre” and “Post” represent pre- and post-EMR exposure measurements. Error bars represent standard deviations.
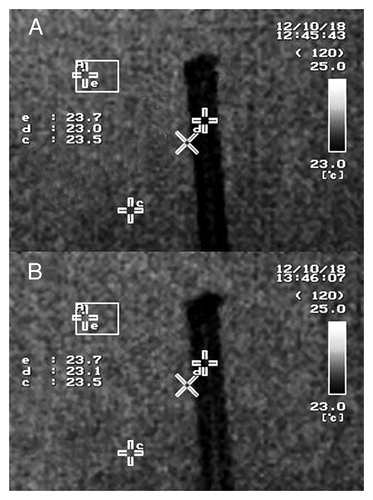
Exposure to EMR did not alter the temperature of plants, even after 1 h continuous exposure. The temperature at a reference point in the EMR exposure chamber and on the plant both increased by 0.1 °C during EMR exposure. The initial temperature of the plant was 23.2 °C, which rose to 23.3 °C after 1 h continuous EMR exposure (). The temperature of the vinyl tube also increased by 0.1 °C after 1 h of continuous exposure, from 23 °C before EMR exposure to 23.1 °C after exposure ().
Figure 3. Thermal images indicating M. aquaticum plant temperatures. (A) Image taken before exposure to 2-GHz radio-frequency electromagnetic radiation (EMR), (B) image taken of the same plant on completion of 1 h continuous EMR exposure. Within each image, the letter “b” indicates the measuring point on the plant canopy, and the letter “e” indicates the temperature measurement position on a 5-mm-thick rigifoam sheet.
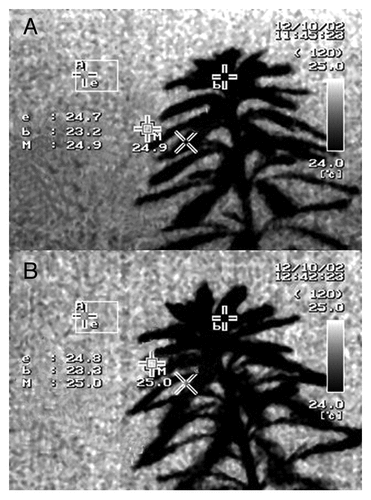
Figure 4. Thermal images indicating the temperature of a vinyl tube filled with distilled water. (A) Image taken before exposure to 2-GHz radio-frequency electromagnetic radiation (EMR), (B) image taken of the same tube on completion of 1 h continuous EMR exposure. Within each image, the letter “b” indicates the measuring point on the plant canopy, and the letter “e” indicates the temperature measurement position on a 5-mm-thick rigifoam sheet.
Discussion
Non-stressed plants at vegetative growth phase can show growth during the light phase.Citation23,Citation24 However, while growth proceeds, there is an observed fluctuation in the length of the plants; this continues even without any measurable overall growth.Citation25 These fluctuations may result from complex mechanistic interactions within plants. For example, an exchange of ions (Ca2+, K+, Cl-) between the symplast and apoplast spacesCitation26 and changes in cell turgorCitation27,Citation28 are needed for long-distance signal transmission through the phloem. This changing cell turgor could cause nanometric fluctuations in plant lengths. Furthermore, the variations in transpiration rate over time can also lead to plant length fluctuations at the micrometer scale,Citation25 and, when the fluctuation in the stem length is measured at 3-mm sections as in the present study, this can be observed at the nanometer scale.
The reduced NERF SDs observed in the present experiment suggest that exposure to 2 GHz EMR caused a change in the physiology of M. aquaticum plants. This level of exposure is less intense than the maximum public exposure guidelines for humans (9.87 Wm-2).Citation5 Reduced NERF can indicate that plants are experiencing stress.Citation21 The observed reduction in NERF could be explained by the accumulation of H2O2 and oxidative stress caused by EMR exposure, as reported by Tkalec, Malaric, and Pevalek-Kozlina;Citation6 Tkalec, Malaric, and Pevalek-Kozlina;Citation9 and Sharma, Singh, Kohli, and Batish.Citation10 Increases in ROS activity after EMR might be due to either an effect on the photosynthetic processCitation29,Citation30 or non-photosynthetic ROS formation in cells,Citation31 but no clear explanation has yet been determined. Oxidative stress and accumulation of ROS in plants negatively affects growthCitation32 and cellular activity, and may lead to cell death.Citation33 Furthermore, if H2O2 were generated as a consequence of exposure of the photosystem to EMR, H2O2 would be expected to induce stomatal closure.Citation34,Citation35 This would lower transpiration rates, explaining the reduction of NERF in EMR-exposed plants. In the current study, NERF was recorded 1.5 h after EMR exposure. The similarity of the NERF SDs for the 2 30-min segments of post-EMR exposure measurement indicates that the plants did not exhibit any recovery, even 2.5 h after exposure. Plants can take 5–18 h to recover from oxidative stress,Citation36,Citation37 and a longer recovery period is therefore likely to be required before restoration of normal NERF patterns can be observed. Future studies should investigate prolonged recovery times after exposure to EMR.
Plants can also be affected by temperature stress,Citation38 and EMR may increase plant temperature by dielectric heating.Citation22 Thermal images indicated that 1 h of continuous exposure to EMR did not alter the temperature of plants. However, physiological processes within the plant may have served to regulate the temperature,Citation39-Citation41 rendering it difficult to assess whether dielectric heating occurred. Nevertheless, the temperature of a vinyl tube filled with distilled water increased by only 0.1 °C after 1 h EMR exposure at a power density more than 4-fold that used for the NERF experiment, suggesting minimal dielectric heating effects. As the EMR power density was lower in the NERF experiments (1.42 Wm−2) than for the temperature experiments (6 Wm−2), any temperature increase in M. aquaticum during EMR exposure in the NERF experiment should have been less than 0.1 °C. An increase of at least 1 °C is required to generate a thermal effect in biological tissue.Citation42 Therefore, in the present study, M. aquaticum plants did not experience heat stress caused by EMR exposure.
Plant growth is stimulated by exposure to stationary magnetic fields or very low frequency electromagnetic fields.Citation14,Citation15,Citation43,Citation44 However, calculations using conversion formulas for continuous wave (CW) EMR reveal that the maximum magnetic flux density was approximately 5.9 × 10−2 µT (Magnetic flux density (µT) = Wm–2/239). This magnetic flux density is relatively small when compared with the magnetic flux densities in the above plant growth experiments (1.5 µT–0.5 T) and the magnetic flux density of the earth’s magnetic field (around 50 µT).Citation45 Therefore, the magnetic flux density of EMR in the present study was unlikely to directly affect the plants. However, the very high frequency of the EMR magnetic field used in the present study might have had a hitherto unknown effect on plant growth. Although the magnetic field of EMR is the same as the electric field of EMR (Electric field (Vm−1) or Magnetic field (Am−1)=) (1), most researchers consider the effects of EMR on plants in terms of the electric field and the effects of dielectric activity. However, because the reported effects of EMR on plants are non-thermal, we cannot determine whether the observed effects were caused by the high-frequency EMR electric field or the magnetic field, and this should be studied further.
Plants adapt to their growing environment.Citation46-Citation48 Plants may thus have adapted to EMR exposure as they are continuously exposed to EMR in the natural environment due to the continuous operation of communication transmitters. Therefore, it is important to study plants exposed to EMR for long periods of time to understand any mechanisms used by plants to adapt to EMR exposure. However, plants have different levels of sensitivity to EMR, depending on the frequency and power density to which they are exposed,Citation49,Citation50 and so it is expected that plants growing in the natural environment would vary in their levels of adaptation according to location and local EMR intensity.
The power density of EMR due to mobile phone base stations (8.6 mWm−2)Citation51 is lower than the power density used in the present study and in most of the studies examining the effects of EMR on plants that are cited in this article. However, increasing power densities can be expected in the future due to the congested deployment of base stations and public exposure guidelines for frequencies above 2 GHz that are as high as 9.87 Wm-2.Citation5 The current public exposure guidelines are based on the thermal effects on biological tissues caused by dielectric heating due to EMR. However, our observations of reduced NERF alongside unchanged temperature, combined with oxidative stress data reported elsewhere, strongly suggest that plants experience non-thermal stress in response to EMR exposure.
Materials and Methods
Plant preparation
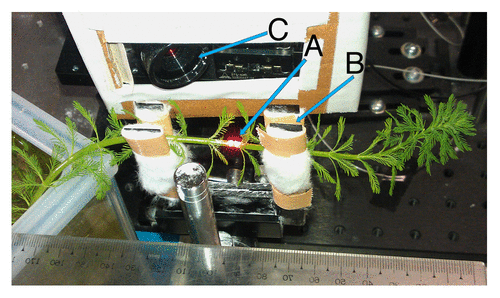
M. aquaticum stems were selected that were at least 15 cm in total length, had emergent portions at least 10 cm in length, and had roots developing on the stem in the submerged portion. The selected stems were cut from the plant stock, and 3–5 stems were planted in clean cube-shaped (15 × 15 × 15 cm) small glass tanks that had been filled to the halfway mark with cleaned fine river sand (sand was thoroughly washed 3 times with tap water and once with distilled water before use). Hoagland solution (35%) was then added to a level of 5 cm above the sand. Water levels in the tanks were maintained by adding distilled water every 2–3 d and 35% Hoagland solution once per week. Plants were grown under 55–60 µmol m−2 s−1 photosynthetically active radiation as measured at 10 cm above the tank, with a 12/12 photoperiod. Stems were allowed to grow until they naturally fell to a horizontal position from their original vertical position (around 20-cm emergent stem length), after which they were used for SIT experiments (). Horizontally positioned plants were used for the experiment because the probing laser beams of the SIT system were arranged horizontally to measure stem elongation.
Statistical interferometry technique
SIT is a novel optical method to measure the strain or change of an object having a rough surface. Statistical interferometry has been successfully employed to measure extremely short-term changes at the sub-nanometer scale in the lengths of plant tissues with the aim of estimating environmental conditions. Therefore, SIT can be used to measure changes in plant elongation and rates of elongation more accurately than can be achieved using conventional techniques.
To achieve measurements of an extremely high accuracy, SIT employs a simple optical system and involves no contact with the studied object. Two points on the stem of the plant under investigation were illuminated by laser beams. As a result of the strong scattering of the sample, 2 speckle fields were generated in the diffraction field of the system. The 2 fully developed independent speckle fields were superimposed, and the resulting 2 speckle fields observed through a charge-coupled device (CCD) camera placed in the diffraction field.Citation52,Citation53 Elongation was determined every 0.5 s. The elongation rate (nm mm−1 sec−1) was obtained from the elongation over a period τ of 5.5 s and was defined by Elongation rate = Elongation / τd where d is laser beam separation. Detailed descriptions of SIT are given in Kobayashi and KadonoCitation21, Kadono, Bitoh, and Toyooka.Citation52 Data were acquired over the whole duration of 1 h.
The NERF measurement and EMR exposure
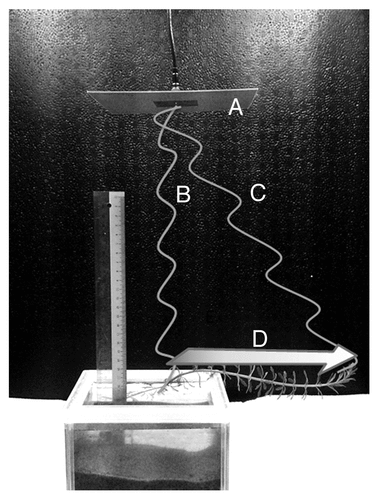
NERF measurements were performed on EMR-exposed and control plants in the same laboratory environment. Environmental EMR exposure was not controlled in either the test or control plants. The stem of each plant was gently clamped, and loose cotton was placed around each plant stem to prevent slippage away from the laser measurement probes. Plants were provided with 55–60 µmol m−2 s−1 photosynthetically active radiation density from a halogen lamp. After a 1.5 h stabilization period, initial NERF were measured continuously for 1 h. Fluctuations were measured 8–10 cm below the top (canopy area) of each plant. After the initial measurements, plants were placed directly under an EMR antenna in an EMR exposure chamber. “EMR-exposed” plants were then subjected to 2-GHz continuous wave (CW; electromagnetic waves of constant amplitude and frequency) EMR at 1.42 Wm−2 power density for 1 h. The power density was approximately 0.65 Wm−2 at the canopy area of the plant, which was approximately 20 cm away from the center of the antenna (). Electromagnetic waves consist of a magnetic and an electric field oscillating perpendicular to one another. In this experiment, the electric field was orientated with the object, and thus EMR polarization was perpendicular to the stem during exposure. Control plants were left in the EMR exposure chamber for 1 h in the absence emission of EMR from the antenna. After 1 h, plants were removed from the EMR exposure chamber and again positioned within the NERF measuring system. After a 1.5 h stabilization period, NERF measurements were made at the plant location where the initial measurements were taken. The experiment was repeated 4 times.
Figure 6. Perpendicular positioning of an M. aquaticum stem with respect to an electromagnetic radio-frequency antenna, resulting a gradient of power intensity along the stem. (A) Transmission antenna, (B) direct waves causing highest power density, (C) angled waves causing low power density, and (D) radio-frequency electromagnetic radiation power density decreases in the direction of the arrow.
Thermographic imaging
Exposure to EMR can increase the temperature of biological materials due to dielectric heating. Dielectric heating is a mechanism by which heat is generated due to the interaction of the alternating electric field of EMR and dipolar water molecules.Citation54 Temperature changes in plants exposed to EMR were assessed using infrared thermographic images with a thermal sensitivity of 0.08 °C (at 30 °C; InfReC Thermo GEAR G120, NEC). In this experiment, 10-cm-tall vertically growing M. aquaticum plants were used. Plants were exposed to 2 GHz CW EMR at a power density of 6 Wm−2 at the canopy area for 1 h. Thermal images of the plant canopy were obtained prior to exposure and immediately before the exposure ended, 1 h later. To investigate the ability of EMR to increase the temperature of a water column with approximately the same diameter as an M. aquaticum stem, a vinyl tube filled with distilled water was subjected to EMR exposure and the changes in temperature measured. In this experiment, a 12-cm vinyl tube that had internal and external diameters of 5 mm and 6 mm, respectively, was filled with distilled water. Both ends of the tube were sealed using silicon sealant (Senedainn silicon sealant 8060). The tube was then positioned vertically, directly under an EMR transmission antenna, by connecting it to a podium. The tube was exposed to EMR of the same frequency and power density as used in the plant thermographic imaging experiments, and thermal images were taken prior to continuous exposure to EMR for 1 h, and immediately before the exposure ended. Thermal measurements of the vinyl tube were targeted 1.5–2 cm below the top of the tube.
Data analysis
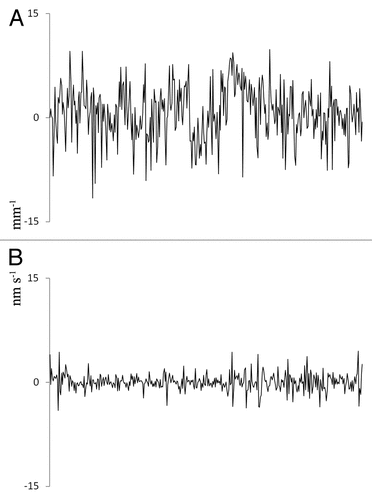
The NERF of the M. aquaticum stem continued over time (). The power spectrum analysis was not able to clearly differentiate the effect of NERF, and thus the NERF standard deviation was calculated to characterize the fluctuations. NERF SDs of EMR-exposed and control plants were compared using 2 independent sample t-tests with the Levene test for equality of variances. Analyses were performed using SPSS 16.0 (SPSS Inc, USA). Thermal images were manually compared with evaluate changes in temperature.
Figure 7. Representative fluctuation of elongation rate of M. aquaticum for 1 h. (A) Nanometric elongation rate fluctuation before radio-frequency electromagnetic radiation (EMR) exposure, and (B) nanometric elongation rate fluctuation after EMR exposure. Vertical axis denotes rate of fluctuation in nm s−1 mm−1 distance of the plant stem. Horizontal axis denotes duration.
| Abbreviations: | ||
| CCD | = | charge-coupled device |
| CW | = | continuous wave |
| EMR | = | radio-frequency electromagnetic radiation |
| NERF | = | nanometric elongation rate fluctuation |
| NERF SD | = | standard deviation of nanometric elongation rate fluctuation |
| SIT | = | statistical interferometry technique |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Prof Yuichi Kimura for helpful discussions during the preparation of this manuscript, Dr Makoto Miwa for valuable support during this study, and Mr Sakuyoshi Saito for his support in the preparation of the EMR exposure system. This study was funded by the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
- Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 2003; 137:65 - 83; http://dx.doi.org/10.1016/S0378-4274(02)00381-8; PMID: 12505433
- Berg M, Stengel C, Pham TK, Pham HV, Sampson ML, Leng M, Samreth S, Fredericks D. Magnitude of arsenic pollution in the Mekong and Red River Deltas--Cambodia and Vietnam. Sci Total Environ 2007; 372:413 - 25; http://dx.doi.org/10.1016/j.scitotenv.2006.09.010; PMID: 17081593
- Chameides WL, Xingsheng L, Xiaoyan T, Xiuji Z, Luo C, Kiang CS, et al. Is ozone pollution affecting crop yields in China?. Geophys Res Lett 1999; 26:867 - 70; http://dx.doi.org/10.1029/1999GL900068
- Hyland G.. How Exposure to Mobile Phone Base-station Signals can Adversely Affect Humans. 2005
- International Commission on Non-Ionizing Radiation Protection. ICNIRP statement on the “Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz)”. Health Phys 2009; 97:257 - 8; http://dx.doi.org/10.1097/HP.0b013e3181aff9db; PMID: 19667809
- Tkalec M, Malarić K, Pevalek-Kozlina B. Exposure to radiofrequency radiation induces oxidative stress in duckweed Lemna minor L. Sci Total Environ 2007; 388:78 - 89; http://dx.doi.org/10.1016/j.scitotenv.2007.07.052; PMID: 17825879
- Senavirathna MDHJ, Asaeda T. Radio-frequency electromagnetic radiation alters the electric potential of Myriophyllum aquaticum. Biol Plant 2013; 1 - 8; http://dx.doi.org/10.1007/s10535-013-0384-3
- Senavirathna MDHJ, Takashi A, Kimura Y. Short-duration exposure to radiofrequency electromagnetic radiation alters the chlorophyll fluorescence of duckweeds (Lemna minor). Electromagn Biol Med 2013; 0:1 - 8; http://dx.doi.org/10.3109/15368378.2013.844705; PMID: 24131393
- Tkalec M, Malarić K, Pevalek-Kozlina B. Influence of 400, 900, and 1900 MHz electromagnetic fields on Lemna minor growth and peroxidase activity. Bioelectromagnetics 2005; 26:185 - 93; http://dx.doi.org/10.1002/bem.20104; PMID: 15768427
- Sharma VP, Singh HP, Kohli RK, Batish DR. Mobile phone radiation inhibits Vigna radiata (mung bean) root growth by inducing oxidative stress. Sci Total Environ 2009; 407:5543 - 7; http://dx.doi.org/10.1016/j.scitotenv.2009.07.006; PMID: 19682728
- Vian A, Roux D, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G. Microwave irradiation affects gene expression in plants. Plant Signal Behav 2006; 1:67 - 70; http://dx.doi.org/10.4161/psb.1.2.2434; PMID: 19521478
- Tingey DT, Reinert RA. The effect of ozone and sulphur dioxide singly and in combination on plant growth. Environ Pollut 1970; 1975:117 - 25; http://dx.doi.org/10.1016/0013-9327(75)90126-3
- Reich PB. Quantifying plant response to ozone: a unifying theory. Tree Physiol 1987; 3:63 - 91; http://dx.doi.org/10.1093/treephys/3.1.63; PMID: 14975835
- Kato R. Effects of a Magnetic-Field on the Growth of Primary Roots of Zea-Mays. Plant Cell Physiol 1988; 29:1215 - 9
- Racuciu M, Creanga D, Horga I. Plant growth under static magnetic field influence. Rom J Physiol 2008; 53:353 - 9
- Murr LE. Plant Growth Response in a Simulated Electric Field-environment. Nature 1963; 200:490 - 1; http://dx.doi.org/10.1038/200490b0; PMID: 14076755
- Takahashi H, Suge H, Kato T. Growth Promotion by Vibration at 50 Hz in Rice and Cucumber Seedlings. Plant Cell Physiol 1991; 32:729 - 32
- Cho U, Park J. Mercury-induced oxidative stress in tomato seedlings. Plant Sci 2000; 156:1 - 9; http://dx.doi.org/10.1016/S0168-9452(00)00227-2; PMID: 10908800
- Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 2001; 52:2115 - 26; http://dx.doi.org/10.1093/jexbot/52.364.2115; PMID: 11604450
- Rathnayake AP, Kadono H, Toyooka S, Miwa M. A novel optical interference technique to measure minute root elongations of Japanese red pine (Pinus densiflora Seibold & Zucc.) seedlings infected with ectomycorrhizal fungi. Environ Exp Bot 2008; 64:314 - 21; http://dx.doi.org/10.1016/j.envexpbot.2008.02.007
- Kobayashi K, Kadono H. Expansion of the dynamic range of statistical interferometry and its application to extremely short- to long-term plant growth monitoring. Appl Opt 2010; 49:6333 - 9; http://dx.doi.org/10.1364/AO.49.006333; PMID: 21068865
- Jacob J, Chia LHL, Boey FYC. Thermal and non-thermal interaction of microwave radiation with materials. J Mater Sci 1995; 30:5321 - 7; http://dx.doi.org/10.1007/BF00351541
- Acevedo E, Fereres E, Hsiao TC, Henderson DW. Diurnal growth trends, water potential, and osmotic adjustment of maize and sorghum leaves in the field. Plant Physiol 1979; 64:476 - 80; http://dx.doi.org/10.1104/pp.64.3.476; PMID: 16660991
- Berman ME, DeJong TM. Diurnal patterns of stem extension growth in peach (Prunus persica): Temperature and fluctuations in water status determine growth rate. Physiol Plant 1997; 100:361 - 70; http://dx.doi.org/10.1111/j.1399-3054.1997.tb04794.x
- Tang AC, Boyer JS. Xylem tension affects growth-induced water potential and daily elongation of maize leaves. J Exp Bot 2008; 59:753 - 64; http://dx.doi.org/10.1093/jxb/erm371; PMID: 18349050
- Lautner S, Grams TE, Matyssek R, Fromm J. Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol 2005; 138:2200 - 9; http://dx.doi.org/10.1104/pp.105.064196; PMID: 16040648
- Fromm J, Bauer T. Action-Potentials in Maize Sieve Tubes Change Phloem Translocation. J Exp Bot 1994; 45:463 - 9; http://dx.doi.org/10.1093/jxb/45.4.463
- Shiina T, Tazawa M. Action-Potential in Luffa-Cylindlica and Its Effects on Elongation Growth. Plant Cell Physiol 1986; 27:1081 - 9
- Krieger-Liszkay A. Singlet oxygen production in photosynthesis. J Exp Bot 2005; 56:337 - 46; http://dx.doi.org/10.1093/jxb/erh237; PMID: 15310815
- Yamasaki H, Sakihama Y, Ikehara N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 1997; 115:1405 - 12; http://dx.doi.org/10.1104/pp.115.4.1405; PMID: 12223873
- Collen J, Delrio MJ, Garciareina G, Pedersen M. Photosynthetic Production of Hydrogen-Peroxide by Ulva-Rigida C-Ag (Chlorophyta). Planta 1995; 196:225 - 30; http://dx.doi.org/10.1007/BF00201378
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci 2004; 9:490 - 8; http://dx.doi.org/10.1016/j.tplants.2004.08.009; PMID: 15465684
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 2004; 55:373 - 99; http://dx.doi.org/10.1146/annurev.arplant.55.031903.141701; PMID: 15377225
- Wang P, Song C-P. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 2008; 178:703 - 18; http://dx.doi.org/10.1111/j.1469-8137.2008.02431.x; PMID: 18373649
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 2004; 55:205 - 12; http://dx.doi.org/10.1093/jxb/erh033; PMID: 14673026
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci 2000; 151:59 - 66; http://dx.doi.org/10.1016/S0168-9452(99)00197-1
- Lehmann M, Laxa M, Sweetlove LJ, Fernie AR, Obata T. Metabolic recovery of Arabidopsis thaliana roots following cessation of oxidative stress. Metabolomics 2012; 8:143 - 53; http://dx.doi.org/10.1007/s11306-011-0296-1; PMID: 22279429
- Lafta AM, Lorenzen JH. Effect of High Temperature on Plant Growth and Carbohydrate Metabolism in Potato. Plant Physiol 1995; 109:637 - 43; PMID: 12228617
- Upchurch DR, Mahan JR. Maintenance of constant leaf temperature by plants—II. Experimental observations in cotton. Environ Exp Bot 1988; 28:359 - 66; http://dx.doi.org/10.1016/0098-8472(88)90060-3
- Seymour RS, Schultze-Motel P. Physiological temperature regulation by flowers of the sacred lotus. Philos T Roy Soc B 1998; 353:935 - 43; http://dx.doi.org/10.1098/rstb.1998.0258
- Watling JR, Grant NM, Miller RE, Robinson SA. Mechanisms of thermoregulation in plants. Plant Signal Behav 2008; 3:595 - 7; http://dx.doi.org/10.4161/psb.3.8.6341; PMID: 19704809
- Ahlbom A, Green A, Kheifets L, Savitz D, Swerdlow A, ICNIRP (International Commission for Non-Ionizing Radiation Protection) Standing Committee on Epidemiology. Epidemiology of health effects of radiofrequency exposure. Environ Health Perspect 2004; 112:1741 - 54; http://dx.doi.org/10.1289/ehp.7306; PMID: 15579422
- Abdul R, Reyad CA, Waleed A, Hussain F. Effects of Magnetic Field on the Growth Development of Zea mays Seeds. Journal of Natural Product and Plant Resources 2012; 2:456 - 9
- Radhakrishnan R, Ranjitha Kumari BD. Pulsed magnetic field: a contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol Biochem 2012; 51:139 - 44; http://dx.doi.org/10.1016/j.plaphy.2011.10.017; PMID: 22153250
- Belyavskaya NA. Biological effects due to weak magnetic field on plants. Adv Space Res 2004; 34:1566 - 74; http://dx.doi.org/10.1016/j.asr.2004.01.021; PMID: 15880893
- Turner N, Begg J. Plant-water relations and adaptation to stress. Plant Soil 1981; 58:97 - 131; http://dx.doi.org/10.1007/BF02180051
- Gomes PIA, Asaeda T. Spatial and temporal heterogeneity of Eragrostis curvula in the downstream flood meadow of a regulated river. Ann Limnol-Int J Lim 2009; 45:181 - 93; http://dx.doi.org/10.1051/limn/2009015
- Asaeda T, Siong K, Kawashima T, Sakamoto K. Growth of Phragmites japonica on a sandbar of regulated river: morphological adaptation of the plant to low water and nutrient availability in the substrate. River Res Appl 2009; 25:874 - 91; http://dx.doi.org/10.1002/rra.1191
- Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, et al. Electromagnetic fields (900 MHz) evoke consistent molecular responses in tomato plants. Physiol Plant 2006; 128:283 - 8; http://dx.doi.org/10.1111/j.1399-3054.2006.00740.x
- Tkalec M, Malarić K, Pavlica M, Pevalek-Kozlina B, Vidaković-Cifrek Z. Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat Res 2009; 672:76 - 81; http://dx.doi.org/10.1016/j.mrgentox.2008.09.022; PMID: 19028599
- Elliott P, Toledano MB, Bennett J, Beale L, de Hoogh K, Best N, Briggs DJ. Mobile phone base stations and early childhood cancers: case-control study. BMJ 2010; 340:c3077; http://dx.doi.org/10.1136/bmj.c3077; PMID: 20570865
- Kadono H, Bitoh Y, Toyooka S. Statistical interferometry based on a fully developed speckle field: an experimental demonstration with noise analysis. J Opt Soc Am A Opt Image Sci Vis 2001; 18:1267 - 74; http://dx.doi.org/10.1364/JOSAA.18.001267; PMID: 11393619
- Kadono H, Toyooka S. Statistical interferometry based on the statistics of speckle phase. Opt Lett 1991; 16:883 - 5; http://dx.doi.org/10.1364/OL.16.000883; PMID: 19776817
- Marra F, Zhang L, Lyng JG. Radio frequency treatment of foods: Review of recent advances. J Food Eng 2009; 91:497 - 508; http://dx.doi.org/10.1016/j.jfoodeng.2008.10.015