Abstract
The ratio of Red to Far Red light (R:FR) is sensed by phytochromes, including phytochrome B, and serves as a signal of potential competition. Low R:FR represses Arabidopsis thaliana branching by promoting the accumulation of abscisic acid in the young buds and by enhancing auxin signaling in the main shoot. While overall plant level branching is reduced by low R:FR, the growth of the uppermost branches tends to be promoted while the lower buds are suppressed. Buds at intermediate positions can show either growth promotion or growth suppression by low R:FR if they become exposed to low R:FR late or early, respectively. This pattern suggests that developmental stage specific programming occurs to modify the response of specific buds to branching regulators including auxin and ABA.
Plant architecture is determined by both intrinsic factors and environmental signals, such as the Red light: Far Red light (R:FR), an example of a classic competition signal that informs the plant about neighboring vegetation.Citation1 An obvious architectural response of plants to reduced R:FR is to suppress bud outgrowth.Citation2-Citation6 In Arabidopsis (Arabidopsis thaliana), buds formed near the top of the rosette have greater outgrowth potential than those further down, and the effects of the R:FR on outgrowth are very dependent on bud position and the timing of exposure.
The R:FR signals are highly dynamic as the ratio can decrease gradually, at a speed that depends on the growth capacity of the neighbors, can increase gradually as a result of the senescence of neighbors, or may increase suddenly as a result of disturbance by wind, herbivores, etc. There is an apparent correlation between the potential asymmetry of the R:FR kinetics and the kinetics of the branching response. High R:FR, which may appear suddenly after low R:FR, rapidly (in less than 24 h) releases the growth of arrested buds.Citation6 However, we have observed that instantaneous exposure to low R:FR, a signal that is normally generated more gradually in natural environments, does not rapidly alter bud outgrowth.
The branching response to the R:FR involves a reshaping of hormonal networks, in particular abscisic acid (ABA) and auxin. Evidence of a role for ABA was generated using a microarray based approach examining the bud transcriptome response to the R:FR. The analysis provided evidence that ABA was involved in regulating the outgrowth response, which was confirmed using biochemical and genetic approaches with the ABA biosynthetic mutants nced3–2 and aba2–1.Citation6 This study focused on the outgrowth response and defined the parameters necessary to generate robust and rapid changes in bud fate that ultimately allowed a role for ABA to be demonstrated. A different study also implied a role for ABA in the regulation of Arabidopsis branching, but the phenotype of a multiple pyr/pyl ABA receptor mutant did not provide conclusive evidence in this regard.Citation7 The contrasting results may be related to the genetic systems used to test the function of ABA. For instance, redundancy in the PYR/PYL family may mask tissue and process specific ABA phenotypes.
The ABA effect was quantitative and was not expected to account for all of the observed bud outgrowth repression under low R:FR. Evidence of a role for auxin was provided using the phytochrome B (phyB) loss of function line that exhibits a phenotype similar to constitutive shade avoidance, including suppressed branching. Defects in phyB branching resulted from elevated auxin signaling, in spite of lower IAA levels in the main stem.Citation8 Thus, both elevated ABA in the axillary buds and elevated auxin signaling associated with the polar auxin transport stream (PATS) likely contribute to the suppression of branching in low R:FR.
To investigate how the timing of low R:FR exposure affects branching, low R:FR was provided to the Arabidopsis Columbia ecotype at various times after sowing, using a growth chamber system fitted with fluorescent lamps and FR light emitting diodes providing a PPFD of 180 μmol m−2 s−1 and a R:FR of 3.52 (high R:FR) or 0.08 (low R:FR). Plants were harvested at 10 d after anthesis and architectural parameters were assessed. Plants exposed to low R:FR beginning 1 d after sowing showed a strong shade avoidance phenotype, while plants exposed to low R:FR later in development, at 7 or 14 d after sowing, showed incrementally reduced responses compared with the high R:FR control (). This pattern is shown, for instance, by the number of branches and leaves, which are reduced by low R:FR (- D). These findings are in agreement with our anecdotal observations that low R:FR has a strong effect on architecture when applied early, but a weak effect when applied late in the life cycle.
Figure 1. (A)Shoot phenotypes, (B) main shoot and rosette branch lengths, (C) number of rosette branches, and (D) number of rosette leaves of WT Col-0 provided with low R:FR after various durations of growth under high R:FR, measured at 10 d after anthesis. Data are means ± SE with n = 16 to 18. Different letters indicate a significant difference (ANOVA, Tukey’s HSD) between high R:FR and low R:FR treatments at α = 0.05. MS = main shoot, Rn = rosette branch n. Arrows in (B) indicate branch Rn-3.
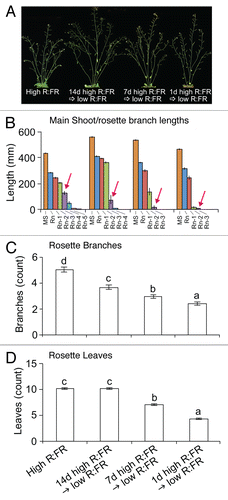
When the analysis is focused on the specific buds the scenario becomes more complex. As reported previously, low R:FR exerted dual effects on branch development.Citation4,Citation6 Low R:FR inhibited the outgrowth of buds from lower positions (see Rn-3 in ), but promoted the elongation of branches at upper positions (see Rn in ). It is noteworthy that the growth of buds at an intermediate position (Rn-2) was promoted by late application of low R:FR (i.e, after 14 d high R:FR) but inhibited by early applications (i.e., after 7 or 1 d high R:FR). Therefore, the late application of low R:FR resulted in a more extreme example of the contrasting effects of this signal on branching noted previously: it inhibited the outgrowth or activation of lower buds, but strongly promoted the growth of upper branches which achieved much greater lengths than those maintained under high R:FR.
The divergent effects of the R:FR on branching suggest that the final response depends on the integration of diverse mechanisms controlled by R:FR signals. Two of these mechanisms involve hormonal signals repressing bud outgrowth in response to low R:FR: one related to increased ABA in the bud itself,Citation6 and the other related to elevated auxin signaling in the main shoot.Citation8 The mechanisms involved in the promotion of branch elongation by low R:FR remain to be elucidated, but a direct action of auxin signaling in the branch emerges as likely candidate, because auxin has been implicated in the promotion of stem growth in young Arabidopsis seedlings,Citation9 and auxin signaling promotes the elongation of the main shoot.Citation10,Citation11 Based on the existing evidence, the following model may be proposed. In plants exposed to low R:FR late in development, the upper buds have presumably already committed to outgrowth prior to the perception of the low R:FR signal and therefore escape its inhibitory effects. The low R:FR may then stimulate auxin signaling in the outgrowing branches, thereby enhancing their elongation compared with WT, and further inhibiting the outgrowth of inferior buds. In other words, with age outgrowing branches are postulated to become functionally more like the main shoot.
How auxin signaling in the main shoot interacts with bud ABA levels to repress outgrowth and how these pathways integrate with the putative local branch auxin signaling pathway promoting outgrowth remains unknown. This issue is central to understand the age-dependent effects of the R:FR on bud growth. Auxin signaling in the main shoot could stimulate bud ABA accumulation or sensitivity to repress outgrowth of inferior buds. Alternatively, ABA could act in a pathway parallel to auxin signaling, responding independently to cues that modify branching. Previous studies have demonstrated that exogenous auxin and ABA applied to excised stems have an additive inhibitory effect on branching,Citation12,Citation13 but how these pathways may be connected in planta has not been conclusively shown. Defining how auxin signaling and ABA may interact to regulate branching is a logical direction for future research in this intriguing area of plant biology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NSF grant IOS-0719414 and by Texas AgriLife Research (S.A.F.).
References
- Casal JJ. Shade avoidance. Arabidopsis Book 2012; 10:e0157; http://dx.doi.org/10.1199/tab.0157; PMID: 22582029
- Kebrom TH, Burson BL, Finlayson SA. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol 2006; 140:1109 - 17; http://dx.doi.org/10.1104/pp.105.074856; PMID: 16443694
- Kebrom TH, Brutnell TP, Finlayson SA. Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ 2010; 33:48 - 58; PMID: 19843258
- Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ. Phytochrome regulation of branching in Arabidopsis. Plant Physiol 2010; 152:1914 - 27; http://dx.doi.org/10.1104/pp.109.148833; PMID: 20154098
- Su H, Abernathy SD, White RH, Finlayson SA. Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis.. Plant Cell Environ 2011; 34:1986 - 98; http://dx.doi.org/10.1111/j.1365-3040.2011.02393.x; PMID: 21726239
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA. Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol 2013; 163:1047 - 58; http://dx.doi.org/10.1104/pp.113.221895; PMID: 23929720
- González-Grandío E, Poza-Carrión C, Sorzano COS, Cubas P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 2013; 25:834 - 50; http://dx.doi.org/10.1105/tpc.112.108480; PMID: 23524661
- Reddy SK, Finlayson SA. Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiol 2014; 164:1542 - 1550; http://dx.doi.org/10.1104/pp.113.234021; PMID: 24492336
- Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis.. Proc Natl Acad Sci U S A 1998; 95:7197 - 202; http://dx.doi.org/10.1073/pnas.95.12.7197; PMID: 9618562
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 1990; 2:1071 - 80; PMID: 1983791
- Jouve L, Gaspar T, Kevers C, Greppin H, Degli Agosti R. Involvement of indole-3-acetic acid in the circadian growth of the first internode of Arabidopsis. Planta 1999; 209:136 - 42; http://dx.doi.org/10.1007/s004250050615; PMID: 10467040
- Chatfield SP, Stirnberg P, Forde BG, Leyser O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 2000; 24:159 - 69; http://dx.doi.org/10.1046/j.1365-313x.2000.00862.x; PMID: 11069691
- Cline MG, Oh C. A reappraisal of the role of abscisic acid and its interaction with auxin in apical dominance. Ann Bot 2006; 98:891 - 7; http://dx.doi.org/10.1093/aob/mcl173; PMID: 16882681
