Abstract
Plants respond to iron deprivation by inducing a series of physiological and morphological responses to counteract the nutrient deficiency. These responses include: (i) the acidification of the extracellular medium, (ii) the reduction of ferric ion and (iii) the increased transport of ferrous ion inside of root cells. This iron transport system is present in strategy I plants and is strictly regulated; at low iron concentration the responses are induced whereas upon iron supply they are repressed. The mechanisms related with this process has been extensively studied, however, the specific cellular effectors involved in sensing iron deficiency, the cascade of components participating in signal transduction, and the way iron is metabolized and delivered, are yet poorly understood. Recently, it has been proposed nitric oxide (NO) as a signaling molecule required for plant responses to iron deficiency. NO is produced rapidly in the root epidermis of tomato plants that are growing under iron deficient conditions. Furthermore, it was demonstrated that NO is required for the expression and activity of iron uptake components in roots during iron deprivation. Here we propose and discuss a working hypothesis to understand the way NO is acting in plants responses to iron deficiency. We specifically highlight the cross talk between NO and plant hormones, and the interaction between NO, iron and glutathione for the formation of dinitrosyl iron complexes (DNICs). Finally, a potential role of DNICs in iron mobilization is proposed.
Addendum to: Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 2007; 52:949-60.
Iron is an essential mineral nutrient for plant growth and developmental processes.Citation1 Thereby, plants have evolved generating mechanisms finely controlled to maintain iron homeostasis. Thus, all plants except grasses exposed to iron deficiency induce a set of morphological and physiological responses termed strategy I. These responses are characterized by: (i) induction of rhizosphere acidifiction mediated by the activation of H+-ATPase, (ii) enhanced activity and expression of Fe3+-chelate reductase (FRO), (iii) increased expression of Fe2+- transporter (IRT) and, (iv) root hair proliferation.Citation2–Citation5 In addition, it has been identified an essential gene in Arabidopsis thaliana, FIT1, which encodes a transcription factor that regulates iron uptake responses.Citation6,Citation7
Nitric Oxide (NO) is a signal molecule that participates in multiple and diverse physiological responses in plants.Citation8,Citation9 There are strong evidences that support a previously uncharacterized signalling role for NO mediating iron deficiency responses in roots.Citation10,Citation11 In a recent report, Graziano and LamattinaCitation11 have demonstrated that accumulation of FRO1, IRT1 and FER (ortholog of Arabidopsis FIT1) mRNAs, increased activity of FRO and root hair proliferation are modulated by NO in tomato (Solanum lycopersicum) seedlings growing under iron deprived conditions. Furthermore, it has been established that NO production increases in roots of iron deficient tomato seedlings just in the most active region of iron uptake.Citation11
It has also been shown that the exogenous application of NO rescued tomato seedlings from the symptoms and phenotype associated to iron deficiency. The NO-induced restoration of normal growth in tomato plants was accompanied by an enhanced expression of responses triggered by the iron deficient conditions.Citation11 Even though, these results are promising evidence supporting a significant function of NO in plant responses to iron deprivation, no data is available on the molecular basis of the NO targets and action. In addition, there are no reports on the way cells perceive and sense the iron deficiency nor on the nature of signal that communicates iron deficiency between cells. Finally, there are no conclusive experimental data on the way iron is transported and delivered in plants.
NO Plays a Role in the Iron Mobilization and Availability
In animals cells, it is well known that NO forms intracellular complexes which play important functions in biological processes.Citation12 For instance, S-nitrosothioles and dinitrosyl iron complexes (DNICs) were proposed as the main forms for NO carriers and storage. DNICs are formed by the interaction between NO and Fe2+ with thiols-containing ligands. NO releasing agents showed high efficacy in iron mobilization from cells.Citation12 It has been demonstrated that NO can stimulate cellular iron and glutathione (GSH) release by a mechanism mediated by a multidrug resistance-associated transport 1 (MRP1).Citation12 Interestingly, electroparamagnetic resonance spectroscopy demonstrated that the DNICs peak in NO-treated cells was increased by MRP1 inhibitors, indicating inhibited DNICs transport from cells.Citation12 These results suggest that NO may mediate iron and GSH efflux from animals cells, by the GSH transporter MRP1, possibly through the formation of DNICs.
However, in comparison, little is known about how the NO participates in iron homeostasis and mobilization in plants. One possibility is that NO promotes iron mobilization as it has already been reported in animals cells.Citation13 According to this, it has been observed that the levels of GSH enhances in sugar beet roots during iron deficiencyCitation14 and the formation of DNICs increased in China rose leaves treated with gaseous NO.Citation15 In Arabidopsis, an ATP binding cassette transporter, AtMRP2, has been characterized as a transporter of GSH S-conjugates.Citation16 Moreover, evidence support that the GSH synthesis is regulated by NO in Medicago truncatula roots.Citation17 Altogether, these results indicate that NO could be mediating an increase in the available iron through DNICs formation. The NO mediated increase of iron mobilization from the roots to leaves, allows them to improve basal metabolism as photosynthesis and reach normal growth conditions even at low iron supply.
NO Regulated Pathways During Iron Deficiency Responses
Auxin and ethylene have been proposed as components of the plant adaptive mechanisms displayed to cope with low iron availability. Arabidopsis mutants defective in auxin or ethylene metabolism as well as experiments made with auxin or ethylene antagonists have demonstrated that these hormones are involved in root hair proliferation during iron deficiency.Citation18 Auxins are not required, apparently, for the induction of Fe3+ chelate reductase activity.Citation19 Results obtained in our laboratory support NO as an active component operating downstream of auxin in the control of root growth and developmental processes. The auxin-induced formation of adventitious roots, lateral roots and root hair proliferation were promoted by the addition of exogenous NO and were prevented by application of the specific NO scavenger, cPTIO.Citation20–Citation23 In addition, it was demonstrated that auxin treatment resulted in a localized NO production in roots during lateral and root hair formation.Citation21,Citation22 Interestingly, iron deficiency induces NO production in tomatoCitation11 and Arabidopsis roots (unpublished results) in a very precise spatial and temporal pattern. Thus, the numerous evidences on NO and auxin participation in root morphology and the NO requirement for plant responses associated to iron deficiencyCitation11 support the hypothesis that NO and auxin would participate in a concerted action to improve the iron bioavailability. Regarding the ethylene role in iron deficiency responses it has been demonstrated that the synthesis of ethylene increases in plants growing in iron deficiency.Citation24 Ethylene precursors and inhibitors promotes or repress, respectively, the expression and activity of FRO2 and IRT1 of Arabidopsis and FRO1 and IRT1 of tomato plants.Citation25 Also, it has been proposed that the effect mediated by ethylene on FRO and IRT could be due to variations in FIT or FER levels.Citation25
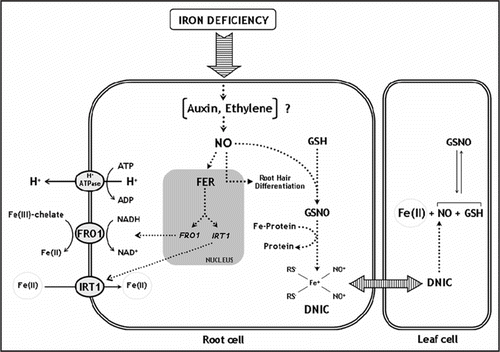
shows a simplified scheme representing the participation of hormones, NO, and DNICs in plants responses to iron deficiency. We postulate NO as an active cellular component involved in iron mobilization and availability in plants alleviating symptoms associated to severe iron deficient conditions. It remains to be determined if NO is also involved in sensing and transducing the iron deficiency signal and if the NO function relies on hormonal action. The transduction of signals controlling perception, uptake, metabolism, storage and delivery of iron is a very hot theme in plants and, as consequence, plant biologists are facing a fascinating time of research in plant nutrition.
Abbreviations
| NO | = | nitric oxide |
| DNICs | = | dinitrosyl iron complexes |
| FRO | = | Fe3+-chelate reductase |
| IRT | = | Fe2+- transporter |
| GSH | = | glutathione |
| MRP1 | = | multidrug resistance-associated transport 1 |
Figures and Tables
Figure 1 Schematic illustration of a model proposing a role for NO, hormones and DNICs during iron deprivation in strategy I plants. Iron deficiency can activate different pathways, probably via the interrelation between NO and hormones (auxin and/or ethylene). NO production is increased during low iron supply in tomato roots. Increased accumulation of FRO1 and IRT1 transcripts results from an NO mediated enhanced FER expression. In this context, iron uptake system in root cells would be activated. In addition, GSH production induced by iron deprivation together with free or protein associated iron in presence of high NO concentration would induce the formation of DNICs in roots cells. These complexes could be a mechanism of iron transport and delivery to others plant cells or organs improving the bioavailability of iron levels.
Acknowledgments
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICTs 38078/05, and 1-14457/03 to L.L.), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP 0898/98 to L.L.) and institutional grants from Universidad Nacional de Mar del Plata (UNMdP), Argentina. L.L. is a member of the research staff, L.R. is a postgraduate fellow and M.G. is a Ph.D. student from CONICET, Argentina.
Addendum to:
References
- Curie C, Briat JF. Iron transport and signaling in plants. Annu Rev Plant Biol 2003; 54:183 - 206
- Schmidt W, Michalke W, Schikora A. Proton pumping by tomato roots: Effect of Fe deficiency and hormones on the activity and distribution of plasma membrane H+-ATPase in rhizodermal cells. Plant Cell Environm 2003; 26:361 - 370
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature 1999; 397:694 - 697
- Eide D, Broderius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 1996; 93:5624 - 5628
- Schmidt W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol 1999; 141:1 - 26
- Colangelo EP, Guerinot ML. The essential basic helix -loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 2004; 16:3400 - 3412
- Bauer P, Ling HQ, Guerinot ML. FIT, the FER-like iron deficiency induced transcription factor in Arabidopsis. Plant Physiol Biochem 2007; 45:260 - 261
- Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G. Nitric oxide: The versatility of an extensive signal. Annu Rev Plant Biol 2003; 54:109 - 136
- Lamattina L, Polacco JC. Lamattina L, Polacco JC. Nitric oxide in plant growth, development and stress physiology. Plant Cell Monographs 2007; Springer
- Graziano M, Beligni MV, Lamattina L. Nitric oxide improves internal iron availability in plants. Plant Physiol 2002; 130:1852 - 1859
- Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 2007; 52:949 - 960
- Watts RN, Hawkins C, Ponka P, Richardson DR. Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1. Proc Natl Acad Sci USA 2006; 103:7670 - 7675
- Graziano M, Lamattina L. van Faassen E, Vanin AF. Nitric oxide and dinitrosyl iron complexes: Roles in plant iron sensing and metabolism. Radicals for Life: The Various forms of Nitric Oxide 2007; Elsevier, BV 161 - 169
- Zaharieva TB, Abadía J. Iron deficiency enhances the levels of ascorbate, glutathione, and related enzymes in sugar beet roots. Protoplasma 2003; 221:269 - 275
- Vanin AF, Svistunenko DA, Mikoyan VD, Serezhenkov VA, Fryer MJ, Baker NR, Cooper CE. Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. J Biol Chem 2004; 279:24100 - 24107
- Lu YP, Li ZS, Drozdowicz YM, Hortensteiner S, Martinoia E, Rea PA. AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione s-conjugates and chlorophyll catabolites: Functional comparisons with AtMRP1. Plant Cell 1998; 10:267 - 282
- Innocenti G, Pucciariello C, Le Gleuher M, Hopkins J, de Stefano M, Delledone M, Puppo A, Baudouin E, Frendo P. Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 2007; 225:1597 - 1602
- Schmidt W, Schikora A. Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiol 2001; 125:2078 - 2084
- Schmidt W, Tittel J, Schikora A. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol 2000; 122:1109 - 1118
- Pagnussat G, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 2003; 132:1241 - 1248
- Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004; 218:900 - 905
- Lombardo MC, Graziano M, Polacco J, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 2006; 1:28 - 33
- Correa-Aragunde N, Lanteri ML, García-Mata C, ten Have A, Laxalt AM, Graziano M, Lamattina L. Lamattina L, Polacco JC. Nitric oxide functions as intermediate in auxin, abscisic acid and lipid signaling pathways. Nitric Oxide in Plant Growth, Development and Stress Physiology 2007; Berlin-Heidelberg Springer-Verlag 113 - 129
- Romera FJ, Alcántara E, De la Guardia MD. Ethylene production by Fe-deficient roots and its involvement in the regulation of Fe-deficiency stress responses by strategy I plants. Ann Bot 1999; 83:51 - 55
- Lucena C, Waters BM, Romera FJ, García MJ, Morales M, Alcántara E, Pérez-Vicente R. Ethylene could influence ferric reductase, iron transporter, and H+-ATPase gene expression by affecting FER (or FER-like) gene activity. J Exp Bot 2006; 57:4145 - 4154