Abstract
Jasmonic acid (JA) is a lipid-derived plant hormone that mediates diverse biological phenomena. It is one of major goals in JA research field to elucidate the regulatory mechanism of JA level. Recently, we have demonstrated cooperative and differentiated roles of two chloroplast localized galactolipases, DGL (DONGLE) and DAD1 (DEFECTIVE IN ANTHER DEHISCENCE 1), for the regulation of JA content. The DGL maintains a basal level of JA in unwounded vegetative tissues, while the DAD1 is involved in JA production in floral tissues. The JA in vegetative tissues regulates cell expansion while the JA produced in flowers regulates pollen maturation. After wounding, the cooperative function of DGL and DAD1 causes drastic increase of JA. The analysis of induction kinetics showed that the two enzymes have temporally separated roles in wound response; DGL in early phase and DAD1 in late phase of JA production. In this addendum, we discuss the implications of our recent findings and extend our working model for JA homeostasis in plants.
Addendum to: Hyun Y, Choi S, Hwang HJ, Yu J, Nam SJ, Ko J, Park JY, Seo YS, Kim EY, Ryu SB, Kim WT, Lee YH, Kang H, Lee I. Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev Cell 2008; 14:183-92.
Jasmonic acid (JA) and its derivatives, collectively referred to as jasmonates, are lipid-derived plant hormones that are ubiquitous in plant kingdom. These compounds play pivotal roles in diverse plant biological processes, such as seed maturation, viable pollen production, root growth, tendril coiling and defense response to biotic and abiotic stresses.Citation1 Biosynthesis of JA is known to be carried out in two sub-cellular organelles, chloroplast and peroxisome, and enzymes involved in this biosynthetic pathway have been characterized by various studies.Citation2 However, initiation and triggering of JA biosynthesis are long-lasted open questions in JA research field. Through the characterization of activation tagging mutant dongle-D (dgl-D), we have firstly demonstrated that chloroplast localized galactolipase DGL catalyzes an initial step of JA biosynthesis in Arabidopsis.Citation3 The dgl-D, DGL overexpressor mutant, showed dwarf phenotype caused by ectopic increase of JA, and the mutant also exhibited constitutive expression of JA responsive genes and increased resistance to fungal pathogen A. brassicicola. While database analysis revealed that DGL shows structural similarity with DEFECTIVE IN ANTHER DEHISCENCE1 (DAD1), a previously reported JA biosynthetic phospholipase A1, DGL and DAD1 exhibit different spatial expression patterns in normal unwounded condition. As a result, the basal level of JA in leaves is regulated by DGL whereas the JA in flowers is regulated by DAD1. Consistently, the RNAi induced knock-down allele, dgl-i, showed decreased JA level in leaves and larger leaf cell size, suggesting that the specific role of DGL is to regulate vegetative organ growth via maintenance of endogenous JA level in vivo. On the other hand, JA in flowers maintained by DAD1 is shown to synchronize pollen maturation, anther dehiscence and flower opening in Arabidopsis.Citation4
Signaling Cascade During Initiation of Wound Response
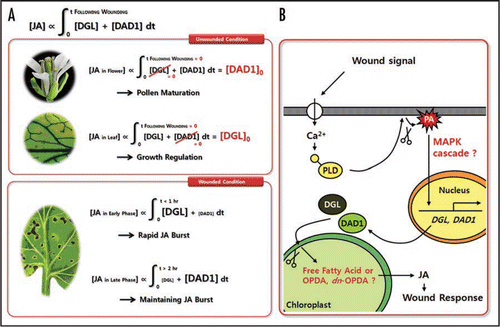
Signals from wounding result in drastic increase of JA level that evokes global scale change of gene expression profile.Citation3,Citation5,Citation6 We have proposed that transcriptional activation of DGL and DAD1 plays pivotal role in wound-induced JA accumulation, based on the lack of JA induction in dgl-i dad1 double mutants.Citation3 Previously, PLDα1 has also been suggested as a key component for wound-induced JA production because PLDα1 antisense Arabidopsis plant showed decreased level of JA production during wound response.Citation7 In our study, we have confirmed that wound-induced expression of DGL and DAD1 is decreased in pldα1 KO but increased in 35S::PLDα1 plants. Therefore, we have proposed that wound-activated PLDα1 generates phosphatidic acid (PA) as a signaling molecule which in turn activates transcription of DGL and DAD1 upon wounding.Citation3 Interestingly, PA-mediated signal transduction for JA production upon wounding has been proposed by several studies. In these studies, rapid increase of cytosolic PA before JA accumulation was shown in wide spectrum of plant species,Citation8,Citation9 and the activation of PLD by wound induced-cytosolic Ca2+ release was further characterized in castor beans.Citation10 In addition, during wound response, the posttranslational activation of MAPK by PA was reported in soybean.Citation11 Hence, findings from these studies suggest that signaling cascade initiated by PA is mediated through MAPKs signaling. Recently, clues supporting this hypothesis were reported in tobacco.Citation12 The RNAi-induced silencing of PA-activated MAPK homologs, WIPK and SIPK, caused defects in JA increase in wounded tobacco plants. In Arabidopsis, it has also been reported that PP2C-type phosphatase AP2C1 negatively regulates MPK4 (WIPK homolog) and MPK6, and modulates jasmonic acid and ethylene level.Citation13 Therefore, it is very likely that wound signals from PLD to DGL and DAD1 are mediated by MAPK cascade.
JA Release from Galactolipids in Chloroplasts
Thylakoid membrane of chloroplasts has a specific feature in lipid composition. In contrast to plasma membranes mainly composed of phospholipids, major component of thylakoid membrane is galactolipid; ∼80% of galactolipid, ∼10% of diacylphosphatidylglycerol, and negligible amount of phospholipid.Citation14 Recently, it was shown that wounding stimulates the rapid accumulation of oxylipin (such as OPDA or dn-OPDA) containing-galactolipids in chloroplast.Citation15 In addition, JA biosynthetic lipoxygenase (LOX) from soybean exhibited substrate specificity to intact phospholipids instead of free fatty acid (linolenic acid),Citation16,Citation17 although LOX2 from barley appears to act on linolenic acid.Citation18 Such results strongly suggest that most of oxylipins, especially OPDA and dn-OPDA, are produced not from free fatty acid, but from acyl groups remaining esterified to galactolipids in chloroplast. Our study showed that DGL has strong and DAD1 has weak galactolipase activity in in vitro assay.Citation3 Hence, DGL and DAD1 may produce free fatty acid from chloroplast membrane. Alternatively, it is probable that they release OPDA or dn-OPDA from galactolipid conjugates as JA precursor. To test this possibility, the substrate specificity of DGL and DAD1 must be analyzed in vivo. In , we present our working model for wound induced JA production pathway with some open questions. Further studies addressing these questions might shed light on understanding the nature of wound signal and JA biosynthetic mechanism.
Abbreviations
| JA | = | jasmonic acid |
| DGL | = | DONGLE |
| DAD1 | = | DEFECTIVE IN ANTHER DEHISCENCE 1 |
Figures and Tables
Figure 1 Schematic Model for JA Generation and Maintenance in Plant. (A) Roles of DGL and DAD1 in JA accumulation are presented as an integral equation. (B) Signaling cascade during initiation of wound response. Wound-induced cytosolic Ca2+ release stimulates translocalization of PLD to plasma membrane. Then, PLD releases phosphatidic acid (PA), and the released PA activates transcription of DGL and DAD1 genes. DGL and DAD1 proteins catalyze chloroplast membrane, and then initiate JA biosynthesis. Our proposed open questions are presented as red letters.
Addendum to:
References
- Creelman RA, Mullet JA. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 1997; 48:355 - 381
- Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell 2002; 14:153 - 164
- Hyun Y, Choi S, Hwang HJ, Yu J, Nam SJ, Ko J, Park JY, Seo YS, Kim EY, Ryu SB, Kim WT, Lee YH, Kang H, Lee I. Cooperation and functional diversification of two closely relate galactolipase genes for jasmonate biosynthesis. Dev Cell 2008; 14:183 - 192
- Ishiguro S, Kawai Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCENCE 1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 2001; 13:2191 - 2209
- Reymond P, Weber H, Damond M, Farmer E. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 2000; 12:707 - 719
- Taki N, Sasaki Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai H, Suzuki H, Masuda T, Takayama K, Shibata D, Kobayashi Y, Ohta H. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 2005; 139:1268 - 1283
- Wang C, Zien CA, Afitlhile M, Welti R, Hildebrand DF, Wang X. Involvement of phospholipase D in wound-induced accumulation of jasmonic acid in Arabidopsis. Plant Cell 2000; 12:2237 - 2246
- Lee S, Suh S, Kim S, Crain RC, Kwak JM, Nam HG, Lee Y. Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J 1997; 12:547 - 556
- Ryu SB, Wang X. Increase in free linolenic and linoleic acids associated with phospholipase D-mediated hydrolysis of phospholipids in wounded castor bean leaves. Biochim Biophys Acta 1998; 1393:193 - 202
- Ryu SB, Wang X. Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochim Biophys Acta 1996; 1303:225 - 243
- Lee S, Hirt H, Lee Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J 2001; 26:479 - 486
- Seo S, Katou S, Seto H, Gomi K, Ohashi Y. The mitogen-activated protein kinases WIPK and SIPK regulate the level of jasmonic and salicylic acids in wounded tobacco plants. Plant J 2007; 49:899 - 909
- Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H, Schwanninger M, Kant M, Schuurink R, Mauch F, Buchala A, Cardinale F, Meskiene I. The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 2007; 19:2113 - 2124
- Douce R, Joyard J. Biochemistry and function of the plastid envelope. Annu Revg Cell Biol 1990; 6:173 - 216
- Buseman CM, Tamura P, Sparks AA, Baughman EJ, Maatta S, Zhao J, Roth MR, Esch SW, Shah J, Williams TD, Welti R. Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiol 2006; 142:28 - 39
- Brash AR, Ingram CD, Harris TM. Analysis of a specific oxygenation reaction of soybean lipoxygenase-1 with fatty acids esterified in phospholipids. Biochemistry 1987; 26:5465 - 5471
- Tojumura A, Sumida T, Toujima M, Kogure K, Fuzukawa K, Takahashi Y, Yamamoto S. Structural identification of phosphatidylcholines having an oxidatively shortened linoleate residue generated through its oxygenation with soybean or rabbit reticulocyte lipoxygenase. J Lipid Res 2000; 41:953 - 962
- Bachmann A, Hause B, Maucher H, Garbe E, Voros K, Weichert H, Waternack C, Feussner I. Jasmonate-induced lipid peroxidation in barley leaves initiated by distinct 13-LOX forms of chloroplasts. J Biol Chem 2002; 383:1645 - 1657