Abstract
Peroxisomes are multifunctional organelles whose abundance and metabolic activities differ depending on the species, cell type, developmental stage, and prevailing environmental conditions.1 However, little is known about the signaling pathways that control these variations, especially in plants. Our laboratory recently investigated the regulatory role of light in changes in peroxisome abundance and identified a phytochrome. A-dependent pathway responsible for the proliferation of peroxisomes during dark-to-light transition in Arabidopsis seedlings. Light induces peroxisome proliferation at least in part through up-regulating the PEX11b gene, which encodes a peroxisomal membrane protein that mediates the early stages of peroxisome multiplication. Activation of PEX11b requires the far-red light receptor phyA, as well as the bZIP transcription factor HYH, which binds directly to the promoter of PEX11b. We conclude that during photomorphogenesis, both the import of leaf-peroxisome enzymes from the cytosol and the induction of peroxisome proliferation take place to prepare seedlings for photosynthesis and photorespiration. In addition to light, other plant peroxisome proliferators may also exert their functions by targeting members of the PEX11 gene family for transcriptional activation.
Addendum to: Desai M, Hu J. Light induces peroxisome proliferation in Arabidopsis seedlings through the photoreceptor phytochrome A, the transcription factor HY5 HOMOLOG, and the peroxisomal protein PEROXIN11b. Plant Physiol 2008; 146:1117-27.
Light is one of the major environmental cues that have profound impacts on plant development. It is perceived by several classes of photoreceptors which initiate a complicated network of downstream signaling events leading to physiological responses including germination, photomorphogenesis, shade avoidance, photoperiodism, phototropism, chloroplast and stomate movement, circadian rhythm and time to flower.Citation2–Citation4 During photomorphogenic responses, Arabidopsis seedlings exhibit hypocotyl growth inhibition, opening of cotyledons, development of chloroplasts and expression of genes involved in photosynthesis and related functions.Citation4 Photoreceptors responsible for seedling photomorphogenesis are phytochromes, which perceive red and far-red light, and cryptochromes, which perceive blue and UV-A light.Citation2–Citation4
What is the role of peroxisomes in photomorphogenesis? Plant peroxisomes are composed of several metabolically specialized subtypes, including glyoxysomes in seeds and germinating seedlings, leaf peroxisomes, gerontosomes in senescent tissue, nodule-specific peroxisomes and unspecialized peroxisomes. Peroxisomes mediate photorespiration, fatty acid β-oxidation, the glyoxylate cycle, nitrogen metabolism, synthesis of plant hormones, metabolism of hydrogen peroxide, and other important physiological processes.Citation5–Citation7 Upon exposure to light, as seedlings undergo the transition from heterotrophic to autotrophic growth, organelles involved in photosynthesis and photorespiration need to develop and genes required in these processes are activated. It is believed that glyoxysomes in dark-grown seedlings are converted into leaf peroxisomes by replacing enzymes specific for lipid metabolism during germination with proteins involved in glycolate recycling during photorespiration.Citation7 To determine whether changes in peroxisome abundance also occur during this process, we analyzed dark-grown Arabidopsis seedlings constitutively expressing the peroxisomal marker protein YFP-PTS1 (Peroxisome Targeting Signal type 1). Upon light treatment, these plants exhibited a strong increase in the total number of peroxisomes in cotyledon cells, which followed a multi-step process consisting of peroxisome elongation, constriction and fission.Citation8
We later identified several components of the light signaling pathway that leads to peroxisome proliferation.Citation8 Previous studies showed that five Arabidopsis PEX11 isoforms, PEX11a to -e, are integral membrane proteins of the peroxisome and promote peroxisome elongation and number increase.Citation9,Citation10 Our study demonstrated that a member of this gene family, PEX11b, was strongly upregulated by light. The RNAi mutant of PEX11b displayed subtle changes in peroxisome morphology and much-reduced increases in peroxisome abundance during dark-to-light transition.Citation8 This finding placed PEX11b as a key peroxisomal mediator in light-induced peroxisome proliferation. To determine which wavelengths of the light spectrum specifically affect the expression of PEX11b, we also performed RT-PCR and searched online gene expression databases to obtain quantitative measures of PEX11b expression under various light conditions. Far-red light was found to confer the strongest upregulation of PEX11b. Consistent with this result, the light induction of PEX11b expression was strongly decreased in mutants impaired in the far-red light receptor phyA. While searching for downstream components of the light signaling pathway that might have an impact on PEX11b expression, we detected a partial but significant reduction in the transcript level of PEX11b in the hyh mutant defective in the bZIP transcription factor HYH.Citation8
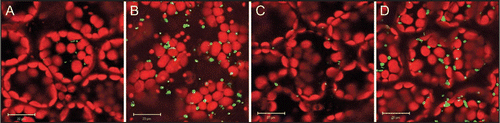
Further support of the role for phyA and HYH in inducing peroxisome proliferation via activating the expression of PEX11b came from our genetic and biochemical analyses. Mutants of PHYA or HYH crossed into the YFP-PTS1 peroxisomal marker line showed significantly reduced peroxisome abundance, a phenotype that was rescued by transiently overexpressing PEX11b in these mutants.Citation8 We subsequently corroborated this transient expression data with confocal microscopic analysis of phyA and hyh mutants stably overexpressing the PEX11b gene (). Interestingly, at the young seedling stage (5d) used in this study, we did not observe many elongated peroxisomes as we did in 6-week adult leaves overexpressing PEX11b.Citation10 indicating that the function of PEX11b and possibly other PEX11 proteins in peroxisome proliferation may be developmentally regulated. Furthermore, gel-shift assays demonstrated that HYH but not its homolog HY5 bound to the promoter of PEX11b, suggesting that PEX11b is one of the distinct target genes of HYH in transcriptional regulation in the light.Citation8
Overall, our study showed that in the complex web of phyA-mediated light signaling cascade, one individual pathway, which is composed of at least the HYH and PEX11b proteins, specifically triggers peroxisome proliferation during seedling photomorphogenesis.Citation8 It seems that in order to keep up with the high rate of photosynthesis and photorespiration, both the import of leaf peroxisomal enzymes into the organelles and a strong increase in peroxisome abundance are required.
Studies have shown that environmental and metabolic stress conditions such as ozone, herbicide, clofibrate and high light increased peroxisome abundance in plant cells.Citation11–Citation16 However, molecular players in the signaling events underlying these phenomena are still elusive. The identification of components in the light signaling pathway that induces peroxisome proliferation in Arabidopsis offers a starting point for dissecting signaling pathways under other peroxisome-proliferating conditions. PEX11 orthologs across kingdoms play highly conserved roles in the rate-limiting first steps of peroxisome multiplication.Citation17 In addition to the light-activated Arabidopsis PEX11b gene, PEX11 from yeast S. cerevisiae and PEX11α from mammals are also transcriptional activation targets for peroxisome proliferation agents such as oleic acid and phenylbutyrate.Citation18–Citation20 The dynamin-related protein DRP3A is another critical player in the Arabidopsis peroxisome proliferation apparatus, which powers the scission of the membranes once constriction occurs.Citation21,Citation22 However, DRP3A does not seem to be a rate-limiting factor and overexpressing the gene did not cause any obvious morphological or population changes of peroxisomes.Citation22 Thus, we predict that besides light, other plant peroxisome proliferators may also use members of the PEX11 gene family as the main entry point to implement their regulatory roles in peroxisome proliferation.
Figures and Tables
Figure 1 Overexpressing PEX11b in phyA and hyh mutants rescued the low peroxisome abundance phenotype. Shown are confocal microscopic images of cotyledon cells from 5-d green seedlings. (A) phyA; (B) phyA overexpressing PEX11b; (C) hyh; (D) hyh overexpressing PEX11b. Scale bars, 20 µm. Peroxisomes are labeled with YFP-PTS1. The bigger and round organelles in the background are chloroplasts emitting autofluorescence.
Addendum to:
References
- Purdue PE, Lazarow PB. Peroxisome biogenesis. Annu Rev Cell Dev Biol 2001; 17:701 - 752
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Ann Rev Genet 2004; 38:87 - 117
- Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet 2007; 8:217 - 230
- Wang H, Deng XW. Dissecting the phytochrome A-dependent signaling network in higher plants. Trends Plant Sci 2003; 8:172 - 178
- Beevers H. Microbodies in higher plants. Ann Rev Plant Physiol 1979; 30:159 - 193
- Nyathi Y, Baker A. Plant peroxisomes as a source of signalling molecules. Biochim Biophys Acta 2006; 1763:1478 - 1495
- Olsen LJ, Harada J. Peroxisomes and their assembly in higher plants. Annu Rev Plant Biol 1995; 46:123 - 146
- Desai M, Hu J. Light induces peroxisome proliferation in Arabidopsis seedlings through the photoreceptor phytochrome A, the transcription factor HY5 HOMOLOG, and the peroxisomal protein PEROXIN11b. Plant Physiol 2008;
- Lingard MJ, Trelease RN. Five Arabidopsis peroxin 11 homologs individually promote peroxisome elongation, duplication or aggregation. J Cell Sci 2006; 119:1961 - 1972
- Orth T, Reumann S, Zhang X, Fan J, Wenzel D, Quan S, Hu J. The PEROXIN11 protein family controls peroxisome proliferation in Arabidopsis. Plant Cell 2007; 19:333 - 350
- Castillo MC, Sandalio LM, Del Rio LA, Leon J. Peroxisome proliferation, wound-activated responses and expression of peroxisome-associated genes are cross-regulated but uncoupled in Arabidopsis thaliana. Plant, Cell Environm 2008; In press
- Nila AG, Sandalio LM, Lopez MG, Gomez M, del Rio LA, Gomez-Lim MA. Expression of a peroxisome proliferator-activated receptor gene (xPPARα) from Xenopus laevis in tobacco (Nicotiana tabacum) plants. Planta 2006; 224:569 - 581
- de Felipe M, Lucas MM, Pozuelo JM. Cytochemical study of catalase and peroxidase in the mesophyll of Lolium rigidum plants treated with isoproturon. J Plant Physiol 1988; 132:67 - 73
- Ferreira M, Bird B, Davies DD. The effect of light on the structure and organization of Lemna peroxisomes. J Exp Bot 1989; 40:1029 - 1035
- Oksanen E, Haikio E, Sober J, Karnosky DF. Ozone-induced H2O2 accumulation in field-grown aspen and birch is linked to foliar ultrastructure and peroxisomal activity. New Phytol 2003; 161:791 - 799
- Palma JM, Garrido M, Rodriguez Garcia MI, del Rio LA. Peroxisome proliferation and oxidative stress mediated by activated oxygen species in plant peroxisomes. Arch Biochem Biophys 1991; 287:68 - 74
- Thoms S, Erdmann R. Dynamin-related proteins and Pex11 proteins in peroxisome division and proliferation. FEBS J 2005; 272:5169 - 5181
- Erdmann R, Blobel G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J Cell Biol 1995; 128:509 - 523
- Li X, Baumgart E, Dong GX, Morrell JC, Jimenez-Sanchez G, Valle D, Smith KD, Gould SJ. PEX11α is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor α-mediated peroxisome proliferation. Mol Cell Biol 2002; 22:8226 - 8240
- Marshall PA, Krimkevich YI, Lark RH, Dyer JM, Veenhuis M, Goodman JM. Pmp27 promotes peroxisomal proliferation. J Cell Biol 1995; 129:345 - 355
- Hu J. Plant peroxisome multiplication: highly regulated and still enigmatic. Int J Plant Biol 2007; 49:1112 - 1118
- Mano S, Nakamori C, Kondo M, Hayashi M, Nishimura M. An Arabidopsis dynamin-related protein, DRP3A, controls both peroxisomal and mitochondrial division. Plant J 2004; 38:487 - 498