Abstract
The aerial organs of plants are covered with a cuticle, a continuous layer overlaying the outermost cell walls of the epidermis. The cuticle is composed of two major classes of the lipid biopolymers: cutin and waxes, collectively termed cuticular lipids. Biosynthesis and transport of cuticular lipids occur predominantly in the epidermis cells. In the transport pathway, cuticular lipids are exported from their site of biosynthesis in the ER/plastid to the extracellular space through the plasma membrane and cell wall. Growing evidence suggests that ATP-binding cassette (ABC) transporters are implicated in transport of cuticular lipids across the plasma membrane of epidermal cells. The Arabidopsis ABC-type transporter protein CER5 (WBC12) was reported to act as a wax monomer transporter. In recent works, our group and others showed that a CER5-related protein, DESPERADO (DSO/WBC11), is required for cutin and wax monomers transport through the plasma membrane of Arabidopsis epidermis cells. Unlike the cer5 mutant, DSO loss-of-function had a profound effect on plant growth and development, particularly dwarfism, postgenital organ fusions, and altered epidermal cell differentiation. The partially overlapping function of CER5 and DSO and the fact that these proteins are half-size ABC transporters suggest that they might form a hetero-dimeric complex while transporting wax components. An intriguing observation was the polar localization of DSO in the distal part of epidermis cells. This polar expression might be explained by DSO localization within lipid rafts, specific plasma membrane microdomains which are associated with polar protein expression. In this review we suggest possible mechanisms for cuticular lipids transport and a link between DSO function and polar expression. Furthermore, we also discuss the subsequent transport of cuticular constituents through the hydrophobic cell wall and the possible involvement of lipid transfer proteins in this process.
Addendum to: Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Höfer R, Schreiber L, Chory J, Aharoni A. The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol 2007; 145:1345-60.
Introduction
Cutin is the largest biopolymer on earth following cellulose and lignin. Along with waxes it is a major component of the cuticle that covers all aerial plant surfaces. Despite the significance of cutin, very little is known about the transport of its monomers from their site of synthesis within epidermal cells to the cuticle construction site in the extracellular matrix. Pighin et al.,Citation1 were the first to provide molecular evidence for the active transport of wax components through the plasma membrane of epidermis cells. Map-based cloning of the Arabidopsis Eceriferum (cer) 5 mutant resulted in the isolation of WBC12, a member of the WBC class in the Arabidopsis ABC-type transporter family. In recent years, plant ABC transporters were implicated in a wide range of biological processes including polar auxin transport, lipids trafficking, xenobiotics detoxification, disease resistance, the regulation of stomatal functions, and transport of secondary metabolites (e.g., alkaloids, terpenoids and phenols).Citation2 Typical to other cer mutants, cer5 exhibits glossy bright stems because of a decrease in epicuticular wax load. Moreover, unusual lipidic inclusions were detected in the mutant stem epidermal cells, suggesting the disruption of cuticular lipids transport from the cytoplasm to the extracellular matrix. Promoter directed expression of a GUS reporter gene indicated stem epidermis specific expression and plasma membrane localization of WBC12-reporter fusion protein (driven by the WBC12 native promoter region). It was therefore suggested that WBC12 acts as a wax transporter. The absence of any additional phenotype in other plant parts apart from stems combined with the absence of changes in cutin composition/levels suggested the existence of additional transporter/s which might act in the delivery of cuticular wax material to the extracellular matrix.Citation1
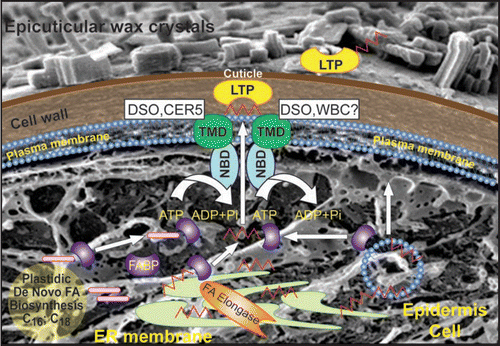
How do waxes and cutin monomers reach the ABC transporter localized in the plasma membrane is also unknown (). Several hypotheses suggest that they might reach the ABC transporter in the plasma membrane through one of the following pathways: (a) binding to an unknown fatty acid binding protein(s) followed by relocation to the transporter; (b) relocation through a vesicular pathway either by the formation of oleosome bodies coated by oleosin-like proteins or the formation of uncoated vesicles that incorporate the cuticular lipids into lipid rafts;Citation3 (c) direct relocation from the ER domains to the close proximity of the plasma membrane; (d) incorporation into lipid rafts sorting pathway through an ER-Golgi-PM route.Citation4 However, there is no direct evidence substantiates any of these hypotheses.
The data gathered thus far suggest that several transporter proteins are involved in the active secretion of lipids for cuticle formation. Recent reports by several research teams including ours on an additional ABC-type transporter involved in cuticular lipids export add another piece to the puzzle. However, many open questions remain regarding the mode of action and interaction of these transporters with the machinery synthesizing and mobilizing cuticular lipids inside the epidermal cells as well as the apparatus that carries them to the cuticle assembly site and specifically to the epicuticular domain.
Results and Discussion
By carrying out a reverse genetics screen of 20 putative Arabidopsis ABC-type transporters belonging to the WBC class we detected an RNAi line [with the DESPERADO (DSO) gene silenced] that exhibited an array of morphological phenotypes.Citation5 The most pronounced phenotypes of the silenced plants were dwarfism, formation of multiple, thin and short inflorescence stems, and severe inter-organ postgenital fusions. The latter phenotype was a first indication for changes in surface structure since similar phenotypes were observed previously in different cuticular mutants. In parallel to our study, the same Arabidopsis gene (At1g17840) was characterized in three other laboratories.Citation6–Citation8 Results from their studies are pointed out in this report. Chemical analysis of various DSO loss-of-function lines revealed that cuticular lipids load, both of wax and cutin constituents, was altered. Results of DSO characterization experiments provide evidence that it is required for both wax and cutin transport carried out by epidermis cells for cuticle assembly. In contrast to the CER5 promoter-reporter assays, the DSO promoter-reporter gene expression was not confined to the epidermal layer, suggesting that it might be involved in the transport of other, cuticular-lipids-like compounds.
While full size ABC transporters consist of two types of core domains, i.e., two nucleotide binding folds (NBFs) which bind and hydrolyze ATP and two transmembrane domains (TMDs),Citation9 half-size transporters (such as DSO) contain a single TMD and one NBF domain. These proteins should therefore form homo- or heterodimers in order to gain functionality.Citation10 One possible candidate to dimerize with DSO for wax transport is the CER5 protein, although two other proteins, belonging to the same phylogenetic clade (WBC15 and WBC13),Citation11 might also act as partners for the transport of cutin and similar type of molecules. For example, in Drosophila, dimerization of three half-transporter ABC proteins related in sequence, White with Brown and White with Scarlet, is required for the transport of different eye pigment precursors into pigment cells.Citation12 Bird et al.,Citation6 generated a double homozygous wbc12/wbc11(dso) mutant and could not detect a difference in wax load between the single mutant alleles and the double mutant. This suggested that these two proteins act in the same pathway and that heterodimers of the two proteins might form a functional complex for wax transport. Yet, attempts to isolate a plasma membrane localized cuticular lipids transport complex and the demonstration of WBC12-WBC11 protein-protein interaction are in progress in several labs.
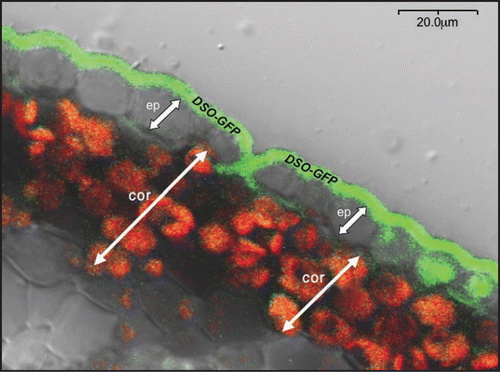
As in the case of cuticular lipids, vectorial transport of substrates requires a polarly expressed transporter. In the course of studying the DSO protein, we found that, following expression of a DSO-GFP-reporter fusion driven by the DSO native promoter, GFP fluorescence was restricted to the distal part (the cuticle side) of stem epidermis cellsCitation5 (). How is DSO differentially distributed between apical and distal parts of the plasma membrane of epidermal cells?. For polar protein expression epidermal cells should be able to establish distinct membrane domains such as lipid rafts. These distinct regions would receive and retain newly synthesized transport proteins in their appropriate sites of functional location.
The link between polar expression and the biological function of transport proteins was reported in several cases in plants. These include the Arabidopsis PIN proteins,Citation13 rice silicon influx and efflux transporters,Citation14,Citation15 and the Arabidopsis boron efflux transporter.Citation16 The transport of auxin in a polar manner along the shoot-root axis requires efflux of carriers such as PIN1. Coordinated polar localization of PIN1 is regulated by Rho-related GTPases (ROP) and the ROP-interactive CRIB motif-containing proteins by mediating two antagonistic pathways with an opposing action on cell polarization. ROP proteins appear to interact directly with upstream regulators of the ARP2/3 complex, which are conserved modulators of the actin cytoskeleton. Their function is associated with polar targeting of PIN efflux transporters to the plasma membrane through the vesicle transport machinery.Citation17 Thus, polarity of the auxin efflux carrier may be established through localized secretion of Golgi vesicles, whereas an attachment of a subunit of the efflux carrier to the actin cytoskeleton may maintain this localization. Future experiments are needed to examine the importance of the various factors influencing PIN proteins transport (e.g., vesicle transport and actin cytoskeleton) for the polar localization of the DSO protein in the plasma membrane.
The mechanism responsible for the localized secretion of the DSO protein in the plasma membrane remains obscure. However, its final membrane localization can be explained by incorporation into lipid rafts. The existence of lipid rafts in plants has been only recently demonstrated.Citation18 Lipid rafts are distinct plasma membrane microdomains with a unique lipid composition. They are enriched in sterols and sphingolipids and devoid of unsaturated phospholipids. Such unique composition enables them to group certain membrane proteins while excluding others. It is hypothesized that the concentration of proteins representing different classes in lipid rafts might serve as a matrix for the formation of various protein-protein complexes including formation of homo- or heterodimers (reviewed in ref. Citation19). The size of wax monomers might span the size of the plasma membrane suggesting the existence of specialized plasma membrane domains (possibly lipid rafts) which can contain them without compromising the membrane structure. Such unique structures could enable further transport to the extracellular matrix by yet unidentified transfer proteins.Citation4 Sterols comprise one of the core components of lipid rafts in eukaryotes. For example, in plants, the PIN1 and PIN3 auxin transporters are polarly expressed and defective biosynthesis of sterols leads to disruption in their polar localization.Citation20 Disruption of membrane homeostasis of sterols using cholesterol chelators could also lead to misexpression of the polarly localized plasma membrane proteins (reviewed in ref. Citation19). Further experiments are required to verify the association of WBC12 and WBC11 with lipid rafts in the plasma membrane of epidermis cells.
While some progress has been made in understanding of cuticular lipids transport across plasma membrane, the mechanism responsible for their extrusion through hydrophobic cell wall remains unknown. The cuticular lipids in the form of monomers or oligomers might passively travel to the extracellular matrix through yet unidentified channels in the cell wall. Another, more likely possibility is that they are picked up from the plasma membrane by non-specific lipid transfer proteins (nsLTPs). This class of proteins contains hydrophobic N-terminal signal sequences, suggesting that LTPs are targeted to the secretory pathway and ultimately to extracellular locations in the cell wall and cuticle. Their small size makes them perfect candidates for the passage through the highly hydrophobic, pectin-rich cell wall. To date, there has been no evidence proving direct involvement of LTPs in cuticular components transport and these proteins have been linked to cuticle metabolism through indirect evidence, as for example epidermis-specific expression, expression in organs with active cuticle biosynthesis, and involvement in conditions that stimulate wax synthesis, such as drought, light or heavy metal treatment.Citation4
Recently, Heredia-Guerrero et al.,Citation21 (2008), raised the possibility of a non-genetic mechanism for cutin monomers transport from the PM to their polymerization site. They suggested that cutin monomers form vesicles (termed cutinosomes) that could fuse upon accumulation and subsequently trigger the formation of a cutin primer which can be further rearranged for polymerization.
Perspective
The evidence regarding the role of ABC transporters in transport of cuticular lipids from the intra- to the extracellular space is becoming more solid. It remains to be elucidated how different WBC transporters interact for the delivery of lipidic material to the surface. In addition, the mechanism responsible for the polar localization of the DSO protein and for the penetration of lipidic molecules through the cell wall also remains obscure.
Figures and Tables
Figure 1 Transport mechanisms involved in cuticular lipids transport in intracellular and extracellular spaces. TMD and NBF-transmembrane domain and nucleotide binding fold of ABC transporter. FABP-fatty acid binding protein. One possibility is that cuticular lipids upon biosynthesis in the ER or plastid are relocated to the PM localized ABC transporter with the aid of FABP. Second possibility is the transport within the oleosome bodies with subsequent incorporation into the PM lipid rafts. DSO and CER5 might interact in the transport of wax monomers, however, additional ABC transporter might form heterodimer with DSO in the delivery of cutin monomers from intracellular space to the outer surface. From the PM cuticular lipids pass through the cell wall to the polymerization site or they are taken up by the lipid transfer proteins (LTPs).
Addendum to:
References
- Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL. Plant cuticular lipid export requires an ABC transporter. Science 2004; 306:702 - 704
- Yazaki K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett 2006; 580:1183 - 1191
- Schulz B, Frommer WB. A plant ABC transporter takes the lotus seat. Science 2004; 306:622 - 625
- Kunst L, Samuels AL. Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 2003; 42:51 - 80
- Panikashvili D, Savaldi Goldstein S, Mandel T, Yifhar T, Franke RB, Hofer R, Schreiber L, Chory J, Aharoni A. The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol 2007; 145:1345 - 1360
- Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu X, Yephremov A, Samuels L. Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 2007; 52:485 - 498
- Luo B, Xue XY, Hu WL, Wang LJ, Chen XY. An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion. Plant Cell Physiol 2007; 48:1790 - 1802
- Ukitsu H, Kuromori T, Toyooka K, Goto Y, Matsuoka K, Sakuradani E, Shimizu S, Kamiya A, Imura Y, Yuguchi M, Wada T, Hirayama T, Shinozaki K. Cytological and biochemical analysis of COF1, an Arabidopsis mutant of an ABC transporter gene. Plant Cell Physiol 2007; 48:1524 - 1533
- Schulz B, Kolukisaoglu HU. Genomics of plant ABC transporters: the alphabet of photosynthetic life forms or just holes in membranes?. FEBS Lett 2006; 580:1010 - 1016
- Rea PA. Plant ATP-binding cassette transporters. Annu Rev Plant Biol 2007; 58:347 - 375
- Sanchez Fernandez R, Davies TG, Coleman JO, Rea PA. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J Biol Chem 2001; 276:30231 - 30244
- Mackenzie SM, Howells AJ, Cox GB, Ewart GD. Sub-cellular localisation of the white/scarlet ABC transporter to pigment granule membranes within the compound eye of Drosophila melanogaster. Genetica 2000; 108:239 - 252
- Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J. Polar PIN localization directs auxin flow in plants. Science 2006; 312:883
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature 2006; 440:688 - 691
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. An efflux transporter of silicon in rice. Nature 2007; 448:209 - 212
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. Plants tolerant of high boron levels. Science 2007; 318:1417
- Xu J, Scheres B. Cell polarity: ROPing the ends together. Curr Opin Plant Biol 2005; 8:613 - 618
- Borner GH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, Macaskill A, Napier JA, Beale MH, Lilley KS, Dupree P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol 2005; 137:104 - 116
- Bhat RA, Panstruga R. Lipid rafts in plants. Planta 2005; 223:5 - 19
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 2003; 15:612 - 625
- Heredia Guerrero JA, BenÌtez JJ, Heredia A. Self-assembled polyhydroxy fatty acids vesicles: a mechanism for plant cutin synthesis. Bioessays 2008; 30:273 - 277