Abstract
The ubiquitin pathway is emerging as a powerful system that controls the stability of key regulatory proteins. In plants, this pathway plays an important role in controlling several developmental processes, responses to environmental changes and also cell division. Arabidopsis SKP2A is an F-box protein that regulates the stability of the E2FC-DPB transcription factor, a repressor of cell proliferation. Although the function of SKP2A is to recruit targets for degradation, we have shown that SKP2A is also degraded through the Ub/26S pathway and, interestingly, auxin stimulates such degradation. Overexpression of SKP2A positively regulates cell division, increasing the number of cells in G2/M, reducing the level of ploidy and developing higher number of lateral root primordia. In addition, we showed in this report that overexpression of SKP2A increased the survival of Arabidopsis plants when they grown on a medium with high levels of sucrose, likely by maintaining cell division active. Thus, it is likely that SKP2A connects cell division with stress responses.
Addendum to: Jurado S, Díaz-Triviño S, Abraham Z, Manzano C, Gutierrez C, Del Pozo C. SKP2A, an F-box protein that regulates cell division, is degraded via the ubiquitin pathway. Plant J 2008; 53:828-41.
The Ubiquitin Pathway and Cell Division in Plants
In the last years, ubiquitination has emerged as a powerful mechanism that regulates the activity of multiple proteins in plants.Citation1–Citation5 Ubiquitin (Ub) is attached to target proteins in a sequential biochemical cascade that involves an E1, an E2 and an E3 enzyme. Primarily, Ub is activated by an E1 in an ATP dependent manner and then the Ub moiety is transferred to an E2 enzyme (also called Ubiquitin conjugating enzyme). The last step might be divided in two parts: first the target protein is recognized by the E3, recruiting it to the proximity of the E2, and second, the Ub moiety is attached to the target.Citation6 Among the different types of E3 described, the SCF complex is one of the most studied. The SCF complex is composed of 4 protein subunits, CUL1, RBX, ASK1 and an F-box. The first three forms a structural core in which different F-box proteins are assembled to generate diverse types of SCF complexes that might recruit different targets.
The completion of the Arabidopsis and rice genome sequences has revealed that these species have a large number of proteins involved in the Ub-pathway (approximately 5% of the total protein), suggesting that a large number of proteins might be regulated through ubiquitination.Citation7,Citation8 In plants, the Ub-pathway regulates the majority of biological processes, such as plant hormones signaling, light responses, circadian clock regulation or pathogen responses (reviewed in refs. Citation1, Citation2, Citation4 and Citation9). In addition to this, the Ub-pathway also regulates cell division by degrading key regulatory proteins. We have identified two F-box proteins, SKP2A and SKP2B, which share homology with human SKP2, a key regulator of cell division in humans. SKP2A targets two cell division transcription factors E2FC and DPB, which negatively regulates cell proliferation.Citation10,Citation11 In addition, SKP2B also seems to regulate the stability of cell cycle proteins. Recently, it has been shown that SKP2B and the RING Protein RKP degraded the CDK inhibitor KRP1.Citation12 Although SKP2A and SKP2B share a high homology at the level of protein sequence, it has been shown that they have different target specificity since SKP2A did not target KRP1Citation12 and SKP2B did not promote DPB degradation.Citation11
F-box protein represent one of the largest family with more than 700 members in Arabidopsis and rice.Citation7,Citation8,Citation13 However, it is not clear whether all of these predicted F-box proteins are able to function as E3 ligases of Ub. Recently, we have developed a biochemical assay to test this activity of F-box protein. Essentially, the SCFMYC-SKP2A is immunopurified from transgenic plant protein extract and then mixed with recombinant E1 and E2 and Ub in the presence of ATP. This assays showed that SKP2A forms an SCF complex with E3 ubiquitin ligase activity, since SCFSKP2A is able to label different proteins with ubiquitin.Citation5
Auxin Regulates the Stability of Skp2A
We have found that SKP2A is also regulated by degradation through the Ub/26S pathway. Interestingly, we found that auxin regulates the stability of SKP2A since when Arabidopsis seedlings are treated with auxin the amount of MYC-SKP2A protein is reduced in an Ub-dependent manner.Citation5 To corroborate this biochemical data with genetic evidences, we crossed the MYC-SKP2AOE plants with mutants that have affected the auxin response (axr1; axr2 and axr3). Immunoblotting analyses showed that SKP2A slightly accumulated in the axr1 mutant, but surprisingly its level was reduced in the axr2 and axr3 mutants.Citation5 This finding might have different explanations. One might be that SKP2A is only degraded when assembled into a SCF complex. It is well known that CUL1 is not modified with RUB in the axr1 mutants,Citation14 what might limit the assembly of SKP2A into a functional SFC, and therefore, SKP2A is not degraded. On the other hand, axr2 and axr3 mutants might alter the auxin response homeostasis or auxin concentration in the plant, what may affect SKP2A degradation. Something else to consider is that we have found that addition of auxin to the in vitro degradation reaction stimulates the degradation of SKP2A (Jurado S and del Pozo C, unpublished). This result suggests that auxin might directly regulate the stability of SKP2A. However, whether this degradation is similar to that found for the IAA/Aux proteinsCitation15,Citation16 need to be tested.
Skp2A Increases Tolerance to Osmotic Stress
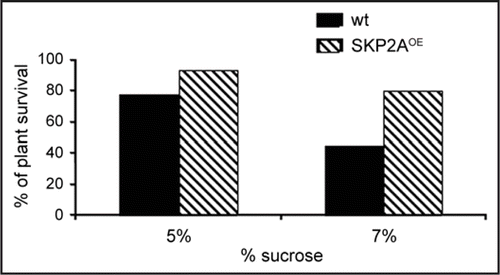
An expression analyses search using public databases revealed that SKP2A is highly induced upon osmotic stress (). To test whether SKP2A has a role during the response to this stress, Arabidopsis wild type and MYC-SKP2A overexpressing seedlings (MYC-SKP2AOE) were grown in a MS medium with increased levels of sucrose. We found that MYC-SKP2AOE plants showed a higher percentage of survival than wild type when grown in a MS medium containing 5 or 7% of sucrose (). This result suggests that SKP2A might have a role during osmotic stress and/or by a stress caused by an excess of sugar. It is known that high level of sucrose in the medium inhibited cotyledon expansion and leaf and root growth.Citation17 We have found that SKP2A overexpression stimulates cell division in the root and shoot.Citation5 Opposite, when Arabidopsis seedlings grown in a medium with high level of sucrose or mannitol, cell division is reduced (our observations). Thus, it is likely that overexpression of SKP2A maintains cell division active longer periods of time that wild type seedlings when they are grown on high amount of sucrose, and therefore SKP2AOE are able to grow more effectively, surviving higher percentage than wt seedlings.
This data and the results published by Jurado and collaborators,Citation5 indicate that SCFSKP2A complex regulates positively cell division by degrading cell cycle repressors. In addition, the fact that MYC-SKP2AOE plants are able to survive better to stresses (high sucrose or phosphate starvation, Jurado S and del Pozo C, unpublished) suggests that SKP2A regulates the programmed cell division but also it might connect cell division to stress responses.
Figures and Tables
Figure 1 SKP2A expression level is increased during osmotic stress. Taken from http://www.bar.utoronto.ca/.18
Figure 2 SKP2A overexpression increased the percentage of plants that survived when grown in high concentration of sucrose. Control and SKP2AOE plants were grown on MS medium with different concentration of sucrose during 14 days. SKP2A overexpression increased plant survival when they are grown in a MS medium with 5% or 7% of sucrose. All plants survived when they were grown in MS medium with 1% or 3% (data not shown).
Addendum to:
References
- Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 2004; 55:555 - 590
- Lechner E, Achard P, Vansiri A, Potuschak T, Genschik P. F-box proteins everywhere. Curr Opin Plant Biol 2006; 9:631 - 638
- Stone SL, Callis J. Ubiquitin ligases mediate growth and development by promoting protein death. Curr Opin Plant Biol 2007; 10:624 - 632
- Dreher K, Callis J. Ubiquitin, hormones and biotic stress in plants. Ann Bot (Lond) 2007; 99:787 - 822
- Jurado S, Diaz-Trivino S, Abraham Z, Manzano C, Gutierrez C, Pozo C. SKP2A, an F-box protein that regulates cell division, is degraded via the ubiquitin pathway. Plant J 2008; 53:828 - 834
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 2002; 82:373 - 428
- Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci 2002; 99:11519 - 11524
- Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, Sharma P, Kapoor S, Tyagi AK, Khurana JP. F-Box Proteins in Rice. Genome-Wide Analysis, Classification, Temporal and Spatial Gene Expression during Panicle and Seed Development, and Regulation by Light and Abiotic Stress. Plant Physiol 2007; 143:1467 - 1483
- Moon J, Parry G, Estelle M. The ubiquitin-proteasome pathway and plant development. Plant Cell, 2004; 16:3181 - 3195
- del Pozo JC, Boniotti MB, Gutierrez C. Arabidopsis E2FC functions in cell division and is degraded by the ubiquitin-SCF (AtSKP2) pathway in response to light. Plant Cell 2002; 14:3057 - 3071
- del Pozo JC, Diaz Trivino S, Cisneros N, Gutierrez C. The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway. Plant Cell 2006; 18:2224 - 2235
- Ren H, Santner A, del Pozo JC, Murray JAH, Estelle M. Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. Plant J 2008; 53:705 - 716
- Risseeuw EP, Daskalchuk TE, Banks TW, Liu E, Cotelesage J, Hellmann H, Estelle M, Somers DE, Crosby WL. Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J 2003; 34:753 - 767
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M. AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis Cullin AtCUL1 is required for auxin response. Plant Cell 2002; 14:421 - 433
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature 2005; 435:441 - 445
- Tan X, Calderon Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007; 446:640 - 645
- Gibson SI. Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol. 2005; 8:93 - 102
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PlosOne 2007; 8:78