Abstract
The cellular and molecular adaptations of non-model woody species to environmental changes are still poorly understood. We have cloned and characterised a novel non-symbiotic hemoglobin from oak roots (QpHb1) which exhibits a specific cellular distribution in the root. The QpHb1 gene is strongly expressed in the protoderm and the protoxylem cells in two Quercus species (Q. petraea and Q. robur) with contrasting adaptive potential to drought and flooding. The constitutive expression of QpHb1 in both oak species in specific root tissues combined with the reported presence of nitric oxide in the same tissues and its potential for protein S-nitrosylation could support a role for non-symbiotic hemoglobins in signalling changes in the root environment and/or in controlling some aspects of root development.
Addendum to: Parent C, Berger A, Folzer H, Dat J, Crèvecoeur M, Badot PM, Capelli N. A novel nonsymbiotic hemoglobin from oak: Cellular and tissue specificity of gene expression. New Phytol 2008; 177:142-54.
Introduction
The genus Quercus (oak) includes over 300 woody species, widespread in the northern hemisphere, where it represents the dominant vegetation of temperate forests.Citation1 Among these, two sympatric predominant European oak species, pedunculate and sessile oak, are known to display different ecological requirements. The two species generally cohabit in forest ecosystems; however, sessile oak is found more frequently on well drained soils, whereas pedunculate oak can populate poorly drained sites.Citation2 This differential spatial distribution is due to the higher tolerance of pedunculate oak for soil waterlogging.
In an attempt to identify pertinent markers to discriminate between the two species, we have cloned and characterised a class 1 non-symbiotic hemoglobin gene. This class of Hbs has a high O2 affinity and is induced under hypoxic conditions.Citation3,Citation4 However, because of an extremely low O2-dissociation constant, class 1 non-symbiotic Hbs may in fact participate in the regulation of cellular nitric oxide (NO) levels thus improving the redox and/or energy homeostasis of plant cells during hypoxia.Citation5,Citation6
Non Symbiotic Hb Shows Differential Distribution in Oak
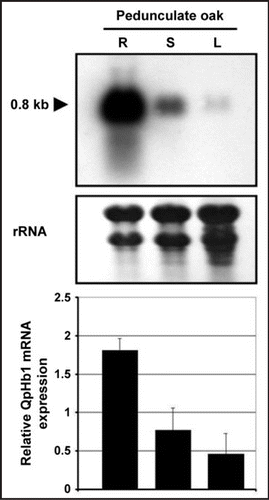
We have recently showed that QpHb1 expression exhibits organ specificity in sessile oak.Citation7 We also found that in pedonculate oak () there is a similar spatial distribution of expression, with a decreasing gradient from roots to leaves. However overall, transcripts are more abundant in pedunculate oak and more specifically in the roots. The fact that ns-Hb is strongly expressed in both species under normal growth conditions, suggests a constitutive role for this protein.
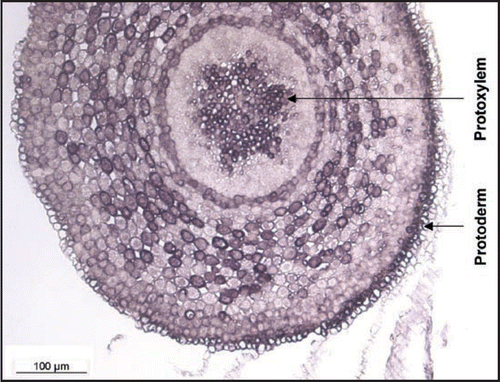
The cellular localisation of QbHb1 in sessile oak roots, as evidenced by in situ hybridization, indicates that hemoglobin transcripts are most abundant in the protoderm and the protoxylem cells. A very similar pattern of expression was also found in pedonculate oak under control conditions (). What could be the significance of such a localisation? Could the constitutive expression of QpHb1 play a role in root development?
A Role for ns-Hb in Root Development and Signalling?
The localisation of ns-Hb in cells undergoing differentiation towards xylem elements but not in protophloem cells, suggests a specific role for ns-Hb in the development of root xylem tissues. Interestingly, plant cells which are just predetermined to irreversibly trans-differentiate in xylem elements, show a burst in NO production.Citation8 This burst is observed when the cells reach a “point of no return” and undergo programmed cell death (PCD), essential to xylem development. The NO/Hb couple is well known to detoxify NO to nitrate via a NAD(P)H dependant mechanism, and thus ns-Hb could play a predominant role for root cell survival by regulating NO levels.Citation9–Citation11 Other authors have also shown a co-localisation of ns-Hb with xylem cells during xylem differentiation in rice.Citation12 It would be extremely interesting to confirm a spatio-temporal co-localisation of ns-Hb expression with the NO burst responsible for protoxylem differentiation.
NO has also been associated with cell signalling through S-nitrosylation of proteins. This post-translational process permits a conformational change of target proteins which can modify their activity.Citation13,Citation14 Recent data suggest that this process is widespread in plants and can be used in regulating various cell processes. Our data showing that ns-Hb is strongly expressed in the root protoxylem could also indicate that the regulation of S-nitrosylation via NO detoxification by ns-Hb or the direct S-nitrosylation of ns-Hb could be key features of root-to-shoot signalling. Indeed, the xylem serves as a direct route between the root and the shoot for various signalling molecules. The constitutive presence of ns-Hb in this tissue may thus serve as a “standby surveillance system” for rapid signalling of changes in the root environment.
Addendum to:
References
- Nixon KC. The genus Quercus in Mexico. Biol Div Mexico 1993; 447 - 458
- Levy G, Becker M, Duhamel D. A comparison of the ecology of pedunculate and sessile oaks: Radial growth in the centre and northwest of France. Forest Ecol Manag 1992; 55:51 - 63
- Duff S, Wittenberg J, Hill R. Expression, purification, and properties of recombinant barley (Hordeum sp.) hemoglobin. Am Soc Biochem Mol Biol 1997; 272:16746 - 16752
- Trevaskis B, Watts R, Andersson C, Llewellyn D, Hargrove M, Olson J, Dennis E, Peacock W. Two hemoglobin genes in Arabidopsis thaliana: The evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA 1997; 94:12230 - 12234
- Dordas C, Rivoal J, Hill R. Plant haemoglobins, nitric oxide and hypoxic stress. Ann Bot 2003; 91:173 - 178
- Perazzolli M, Dominici P, Romero-Puertas M, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 2004; 16:2785 - 2794
- Parent C, Berger A, Folzer H, Dat J, Crèvecoeur M, Badot PM, Capelli N. A novel nonsymbiotic hemoglobin from oak: cellular and tissue specificity of gene expression. New Phytol 2008; 177:142 - 154
- Gabaldon C, Gomez Ros LV, Pedreno MA, Barcelo AR. Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytol 2005; 165:121 - 130
- Perazzolli M, Romero Puertas MC, Delledonne M. Modulation of nitric oxide bioactivity by plant haemoglobins. J Exp Bot 2006; 57:479 - 488
- Dordas C, Hasinoff B, Igamberdiev A, Manac'h N, Rivoal J, Hill R. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 2003; 35:763 - 770
- Igamberdiev A, Bykova N, Hill R. Nitric oxide scavenging by barley hemoglobin is facilitated by a monodehydroascorbate reductase-mediated ascorbate reduction of methemoglobin. Planta 2006; 223:1033 - 1040
- Ross EJH, Shearman L, Mathiesen M, Zhou YJ, Arredondo Peter R, Sarath G, Klucas RV. Nonsymbiotic hemoglobins in rice are synthesized during germination and in differentiating cell types. Protoplasma 2001; 218:125 - 133
- Grennan AK. Protein S-nitrosylation: Potential targets and roles in signal transduction. Plant Physiol 2007; 144:1237 - 1239
- Wang Y, Yun BW, Kwon E, Hong JK, Yoon J, Loake GJ. S-nitrosylation: An emerging redox-based post-translational modification in plants. J Exp Bot 2006; 57:1777 - 1784