Abstract
Ethylene has long been regarded as a stress-related hormone, but only recently the link between ethylene signaling pathway and salt stress was primarily established. Ethylene signaling modulates salt response at different levels, including membrane receptors, components in cytoplasm, and nuclear transcription factors in the pathway. However the relevant mechanism is still unclear. In this paper, we described how ethylene signaling pathway regulates salt stress response and discussed the challenges of ethylene and receptor signaling in salt response regulation.
Introduction
Salinity is one of the most severe stresses among the abiotic stresses and affects crop production of about 23% of cultivated land.Citation1 Plant responses to salt stress is regulated by multiple internal and external factors.Citation1,Citation2 Ethylene, as a gaseous plant hormone, is also involved in plant stress responses, in addition to its roles in germination, fruit ripening, organ abscission, pathogen response and senescence etc.Citation3–Citation5 In the past two-decades, the linear signal pathway of ethylene has been established based on the identification of ethylene-response mutants in Arabidopsis.Citation3,Citation5 Ethylene is perceived by a family of receptors with sequence similarity of bacterial two-component histidine kinase. Downstream of the receptor is CTR1, which is also a negative regulator in the pathway. EIN2 and EIN3 are positive regulators of ethylene response, acting as downstream components of CTR1, and EIN3 mediates transcriptional cascade of ethylene-regulated genes.Citation3–Citation5 Although ethylene is generally believed as a stress-hormone and ethylene signaling regulates multiple stress responses, it is not clear what specific roles the different components of ethylene signaling pathway play in salt stress responses. Only recently, the roles of ethylene signaling in salt stress response were gradually disclosed.Citation6–Citation9 Here, we reviewed and discussed the recent advances about the regulation of plant responses to salt stress by ethylene signaling pathway.
Ethylene Receptor Signaling and Salt Stress Response
Ethylene receptor is divided into two groups according to their sequence similarity and structure characteristics in different plant species. In Arabidopsis, ETR1 and ERS1 belong to the group I subfamily, while ETR2, EIN4, and ERS2 are subfamily II receptors. The expression of ETR1 gene seems to be downregulated by salt and osmotic stress at both transcription and protein levels in Arabidopsis.Citation10 However, many ethylene receptor genes can be induced by multiple stresses.Citation11,Citation12 The gain-of-function mutant etr1-1, with ethylene insensitivity, showed increased sensitivity to salt stress during germination and early seedling development.Citation6,Citation9,Citation13 On the contrary, the strong loss-of-function mutant etr1-7 showed increased tolerance to salt stress.Citation13 The gain-of-function mutants ein4-1 and etr2-1, and Arabidopsis plants with overexpression of dominant mutation of ERS2 (mutation in ethylene binding site in transmembrane domain) also exhibited enhanced sensitivity to salt stress compared to wild type (Li ZG, Chen SY and Zhang JS, unpublished data).Citation6 These analyses indicate that ethylene receptors regulate salt-sensitive response. Studies on a subfamily II ethylene receptor NTHK1 from tobacco demonstrated the similar conclusions. Transgenic Arabidopsis and tobacco plants overexpressing the NTHK1 showed sensitivity to salt stress compared to wild type plants.Citation6,Citation8,Citation9 NTHK1 contains four transmembrane domains, a GAF domain, a kinase domain and a receiver domain. Biochemical studies have revealed its serine/threonine kinase activity.Citation14 The role of different domains of NTHK1 was analyzed in transgenic Arabidopsis plants and it is found that the kinase domain of NTHK1 is required for salt-sensitive epinasty phenotype while the receiver domain is not required for this response. Further mutation of the N-box in kinase domain abolished the NTHK1 kinase activity and resulted in the loss of salt sensitivity in transgenic plants, suggesting that the NTHK1 Ser/Thr kinase activity is essential for the salt sensitive response (Chen T, Liu J, Chen SY and Zhang JS, unpublished data). It is interesting to find that ACC (an ethylene precursor) suppresses the salt sensitivity conferred by NTHK1 in transgenic Arabidopsis plants, suggesting that ethylene is required for counteraction of receptor function to improve salt tolerance. Interestingly, the NTHK1 mRNAs accumulate in both transgenic. Arabidopsis and tobacco plants under salt stress treatment and this accumulation does not require de novo protein synthesis,Citation6,Citation8,Citation9 suggesting that the regulation of the NTHK1 mRNA level follows a posttranscriptional mechanism. Further analysis revealed that the salt-responsive element was located in the transmembrane-coding region but not in the promoter region.Citation9 This phenomenon may indicate a special mechanism for regulation of ethylene receptor genes in response to salt stress.
Ethylene receptors may function in stress response through regulation of salt-responsive gene expressions. Overexpression of the tobacco ethylene receptor gene NTHK1 in Arabidopsis plants promoted expression of AtERF4, Cor6.6, rd17, RD21A, and VSP2, but inhibited expression of BBC1, Lea and AtNAC2 genes.Citation6,Citation9,Citation15 In etr1-1 and ein4-1 gain-of-function mutants, the Cor6.6 was also consistently enhanced. In tobacco plants overexpressing NTHK1, salt-induction of the NtACS1 was suppressed whereas the NTHK1 appears to promote early induction of the NtACO3 gene. Salt-induction of the NtERF1 and NtERF4 was also stronger in NTHK1-transgenic tobacco plants compared to wild type plants.Citation9 Altered downstream gene expressions may contribute to the salt stress response conferred by overexpression or gain-of-function of ethylene receptors.
Arabidopsis subfamily I receptors ETR1 and ERS1 interact with CTR1 and regulate downstream response. However, subfamily II receptors (ETR2, EIN4, and ERS2) appear to have only very weak interactions with CTR1.Citation16,Citation17 Studies also have shown that EIN3 protein turnover remains responsive to ethylene in ctr1 loss-of-function mutant but not in ETR1 loss-of-function mutant etr1-7, and the loss-of-function ctr1 mutant still have a little ethylene response. Moreover, the quadruple mutant of ethylene receptors of Arabidopsis has more severe phenotype than null mutant ctr1. All these facts indicate that another by-pass signaling pathway independent of CTR1 but acting downstream of ethylene receptors may exist. Using a CytoTrap yeast two-hybrid system, we have identified a novel NTHK1-interacting protein (Nip) which associates with tobacco ethylene receptor NTHK1 to mediate salt and other stress responses in transgenic tobacco plant (Cao YR, Chen SY and Zhang JS, unpublished data). Further investigation should shed light on the role of this protein in mediating ethylene receptor signaling and regulation of stress responses.
Other Components of Ethylene Signaling and Salt Stress Response
The functions of other components of ethylene signaling in salt stress response were also investigated. The ctr1-1 mutant exhibited increased salt tolerance, and germination rate and post-germination development of ctr1-1 are more tolerance under salt and osmotic treatments, especially under high concentration of salt stress,Citation7,Citation18 indicating that CTR1 acts as negative regulator to salt stress. EIN2 is a central component of the ethylene signaling transduction pathway in plants, and its null mutant ein2 has complete insensitivity to ethylene at the morphological, physiological, and molecular levels in Arabidopsis.Citation19 Under salt stress, the ein2-1 mutant is severely affected in both seedling growth and in seed germination process, suggesting that EIN2 is required for salt stress tolerance.Citation6,Citation7 It should be mentioned that because ethylene signaling affects seed germination and ethylene-responsive mutant seeds often have enhanced or delayed germination under normal condition, explanation for the roles of ethylene signaling components in germination process under salt and other stresses need to be cautious. The EIN2 may also act as a cross-talk point for multiple signaling pathways involving hormones and stesses.Citation7,Citation19,Citation20 EIN3 is an important component downstream of EIN2 involved in ethylene signaling. As a plant-specific transcription factor, EIN3 regulates multiple ethylene-related gene expression, and binds directly to the promoter region of ERF1 (ethylene-responsive factor 1), and ERF1 then evoke its downstream genes expression. EIN3 degradation is controlled by two F-box proteins EBF1 and EBF2.Citation21,Citation22 The ein3-1 mutants (lacking EIN3) exhibited reduced salt tolerance at higher salt concentration but not at lower concentration and that ebf1-1ebf2-1 mutants exhibited increased salt tolerance.Citation6,Citation18 The increased salt tolerance of ebf1-1 ebf2-1 mutants was abolished by lack of EIN3 in ein3-1 ebf1-1 ebf2-1 mutant plants.Citation18 Overexpression of several ERF genes enhanced salt tolerance in transgenic lines.Citation23,Citation24 However, it should be noted that connection of these ERF genes into ethylene signaling needs to be confirmed and it is possible that some of these ERF genes is not related to ethylene signaling. AtNAC2, another transcription factor regulates lateral roots development and salt response in Arabidopsis, whose gene expression is induced by salt treatment through ethylene signaling and auxin signaling.Citation15 Recently, a novel component MKK9 was found to act downstream of CTR1 to directly modulate EIN3 function in ethylene signaling.Citation25 The mkk9 mutant displayed a broad spectrum of ethylene-insensitive phenotype, including salt stress sensitivity.Citation25
Conclusion and Perspective
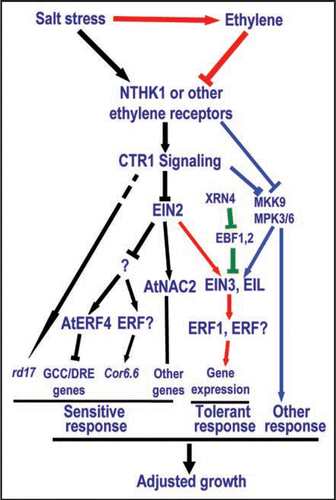
Plants have evolved various specific mechanisms to adapt themselves to the changing environment. Ethylene signaling is one of these pathways that plants have adopted for regulation of salt stress responses. However, many puzzles inside in the ethylene signaling pathway remain to be elucidated. For example, since overexpression or gain-of-function of ethylene receptors results in salt sensitivity, is it possible that these receptors function as sensors for stresses in the absence of ethylene? Because salt stress induces ethylene-biosynthesis genes and ethylene production,Citation6,Citation18 ethylene may then inhibit its receptors, suppress salt sensitivity conferred by ethylene receptors and promote ethylene responses including salt tolerance. Negative regulation between ethylene and its receptors has been established.Citation26 This speculation may explain the fact that salt-stressed NTHK1-overexpressing Arabidopsis plants, with salt sensitive phenotype, can recover after growth for a longer period in plates, possibly due to the accumulation of ethylene in plants or in plates (data not shown). We thus propose a model for the role of ethylene signaling in the adaptive response of plants to salt stress (). Salt tolerance or sensitivity may depend on the balance or homeostasis between ethylene and its receptors in a plant. If receptor function or signaling is intensified, the plants will have large rosette, late flowering but show sensitivity to salt stress.Citation6 If ethylene production or signaling is enhanced, the plants will have small rosette, relatively early flowering but more tolerance to salt stress. Neither of these two extreme situations is beneficial for plant life cycle. In the former case, the plants may have a good vegetative stage but has a risk of producing no or less seeds. In the latter case, the plants may have only very limited energy and resources for production of seeds due to limited vegetative growth. The plants need to make adjustments at various levels for ethylene and receptor signaling to avoid the two above situations and finally can complete the life cycle smoothly or survive safely. The model needs to be tested with further studies.
Signal transduction from ethylene receptors to the downstream components also requires further attention especially at the biochemical level. The difference in the signal output of ethylene receptors in the presence or absence of ethylene is a further challenge for scientists. As a central integrative component of the pathway, EIN2 is a large protein (about 140 KDa) with 12 transmembrane domains in the middle part.Citation19 How this protein accepts signal and transduces signals to other components remains to be an open question. From ethylene receptors to EIN2, new components may be incorporated in addition to CTR1. Some responses exerted by receptors may even not require EIN2. All these studies should be conducted in relation to salt or other stress responses and will certainly advance our understanding of the roles of ethylene signaling pathway in plant response to salt stress.
Abbreviations
| CTR1 | = | constitutive triple response 1 |
| EIN2/3/4 | = | ethylene-insensitive 2/3/4 |
| ETR1/2 | = | ethylene response 1/2 |
| ERS1/2 | = | ethylene response sensor 1/2 |
| NTHK1 | = | Nicotiana tabacum histidine kinase 1 |
| ERF | = | ethylene-responsive factor |
| ACC | = | 1-aminocyclopropane-l-carboxylic acid |
Figures and Tables
Figure 1 Ethylene signaling and salt stress response. Black arrows and lines indicate active receptor signaling, which leads to temporary sensitive response under salt stress through suppression of AtNAC2 and activation of AtERF4. Red arrows and lines indicate active ethylene signaling, which inhibits receptor signaling and leads to tolerant response under salt stress. Green lines and bars indicate the pathway regulating the EIN3 stability. Blue arrows and lines indicate new pathway bypassing EIN2 and regulating ethylene response and salt stress response.Citation25
Acknowledgements
This work was supported by the key project of National Science Foundation of China (90717005) and the National Basic Research Program of China (2006CB100102). We apologize if some references are not cited due to space limits.
References
- Tuteja N. Abscisic acid and abiotic stress signaling. Plant Signaling Behav 2007; 2:135 - 138
- Chinnusamy V, Zhu J, Zhu JK. Salt stress signaling and mechanisms of plant salt tolerance. Genet Eng 2006; 27:141 - 177
- Chen YF, Etheridge N, Schaller GE. Ethylene signaling transduction. Ann Bot 2005; 95:901 - 915
- Chen T, Zhang JS. Ethylene biosynthesis and signaling pathway. Chin Bull Bot 2006; 5:519 - 530
- Guo H, Ecker JR. The ethylene signaling pathway: new insights. Curr Opin Plant Biol 2004; 7:40 - 49
- Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 2007; 143:707 - 719
- Wang Y, Liu C, Li K, Sun F, Hu H, Li X, Zhao Y, Han C, Zhang W, Duan Y, Liu M, Li X. Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Mol Biol 2007; 64:633 - 644
- Cao WH, Liu J, Zhou QY, Cao YR, Zheng SF, Du BX, Zhang JS, Chen SY. Expression of tobacco ethylene receptor NTHK1 alters plant responses to salt stress. Plant Cell Environ 2006; 29:210 - 219
- Zhou HL, Cao WH, Cao YR, Liu J, Hao YJ, Zhang JS, Chen SY. Roles of ethylene receptor NTHK1 domains in plant growth, stress response and protein phosphorylation. FEBS Lett 2006; 580:1239 - 1250
- Zhao XC, Schaller GE. Effect of salt and osmotic stress upon expression of the ethylene receptor ETR1 in Arabidopsis thaliana. FEBS Lett 2004; 562:189 - 192
- Zhang JS, Xie C, Liu F, Liu FH, Chen SY. A novel tobacco gene coding for a product similar to bacterial two-component regualtors. Chin Sci Bull 1999; 44:1025 - 1029
- Zhang JS, Xie C, Shen YG, Chen SY. A two-component gene (NTHK1) encoding a putative ethylene-receptor homolog is both developmentally and stress-regualted in tobacco. Theor Appl Genet 2001; 102:815 - 824
- Wang Y, Wang T, Li K, Li X. Genetic analysis of involvement of ETR1 in plant response to salt and osmotic stress. Plant Growth Regul 2008; 54:261 - 269
- Xie C, Zhang JS, Zhou HL, Li J, Zhang ZG, Wang DW, Chen SY. Serine/Threonine kinase activity in the putative histidine kinase-like ethylene receptor NTHK1 from tobacco. Plant J 2003; 33:385 - 393
- He XJ, Mu RL, Cao WH, Zhang ZG, Zhang JS, Chen SY. AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J 2005; 44:903 - 916
- Clark KL, Larsen PB, Wang X, Chang C. Association of the Arabidopsis CTR1 Raflike kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA 1998; 95:5401 - 5406
- Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. Localization of the Raf-like kinase CTR1 to the endoplasmin reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 2003; 278:34725 - 34732
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science 2006; 311:91 - 94
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 1999; 284:2148 - 2152
- Bouchez O, Huard C, Lorrain S, Roby D, Balagué C. Ethylene is one of the key elements for cell death and defense response control in the Arabidopsis lesion mimic mutant vad1. Plant Physiol 2007; 145:465 - 477
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 2003; 115:679 - 689
- Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 2003; 115:667 - 677
- Wang H, Huang Z, Chen Q, Zhang Z, Zhang H, Wu Y, Huang D, Huang R. Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance. Plant Mol Biol 2004; 55:183 - 192
- Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY, Li LC, Zhao YX, Lu Y, Ni ZY, Liu L, Qiu ZG, Ma YZ. Isolation and molecular characterization of the Triticum aestivum L.ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol 2007; 65:719 - 732
- Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J. Dual control of nuclear EIN3 by bifuracate MAPK cascades in C2H2 signalling. Nature 2008; 451:789 - 795
- Hua J, Meyerowita EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 1998; 94:261 - 271