Abstract
In plant cells, glycans attached to asparagine (N) residues of proteins undergo various modifications in the endoplasmic reticulum and the Golgi apparatus. The N-glycan modifications in the Golgi apparatus result in complex N-glycans attached to membrane proteins, secreted proteins, and vacuolar proteins. Recently, we have investigated the role of complex N-glycans in plants using a series of Arabidopsis thaliana mutants affected in complex N-glycan biosynthesis.1 Several mutant plants including complex glycan 1 (cgl1) displayed a salt-sensitive phenotype during their root growth, which was associated with radial swelling and loss of apical dominance. Among the proteins whose N-glycans are affected by the cgl1 mutation is a membrane anchored β1,4-endoglucanse, KORRIGAN1/RADIAL SWELLING2 (KOR1/RSW2) involved in cellulose biosynthesis. The cgl1 mutation strongly enhanced the phenotype of a temperature sensitive allele of KOR1/RSW2 (rsw2-1) even at the permissive temperature. This establishes that plant complex N-glycan modification is important for the in vivo function of KOR1/RSW2. Furthermore, rsw2-1 as well as another cellulose biosynthesis mutant rsw1-1 exhibited also a salt-sensitive phenotype at the permissive temperature. Based on these findings, we propose that one of the mechanisms that cause salt-induced root growth arrest is dysfunction of cell wall biosynthesis that induces mitotic arrest in the root apical meristem.
Addendum to: Kang JS, Frank J, Kang CH, Kajiura H, Vikram M, Ueda A, Kim S, Bahk JD, Triplett B, Fujiyama K, Lee SY, von Schaewen A, Koiwa H. Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc Natl Acad Sci USA 2008; 105:5933-8.
In eukaryotic cells, both soluble and membrane proteins that enter the endoplasmic reticulum (ER) system may undergo post-translational modifications called N-glycosylation. N-glycosylation occurs in two phases, namely, core glycosylation in the ER and glycan maturation in the Golgi apparatus.Citation2,Citation3 The process and roles of core glycosylation in the ER are well established and ubiquitous for eukaryotes. In the ER, pre-assembled core oligosaccharides (Glc3Man9GlcNac2) are transferred to asparagine residues of the Asn-X-Ser/Thr motives in nascent polypeptides by the function of an oligosaccharyltransferase complex (OST). Terminal glucose residues are recognition sites for ER chaperones calnexin and calreticulin, and thus core N-glycans in the ER function in correct folding of newly synthesized proteins.Citation2,Citation3
Greater diversity exists in the N-glycan maturation steps in the Golgi apparatus and conspicuous roles for the resulting complex N-glycans.Citation2,Citation4 In general, mature N-glycan structures are classified as oligomannosidic type, hybrid or complex type. Glycoprotein precursors that are exported from the ER carry high-mannose type N-glycan intermediates. Numerous enzymes are involved in the conversion of high-mannose type N-glycans to mature complex N-glycans. The functions of N-glycan modifications in the Golgi apparatus are well established in humans, because lack of N-glycan maturation results in Type II Congenital Disorders of Glycosylation.Citation5 In Drosophila melanogaster, the Golgi pathway is necessary for development and function of the central nervous system,Citation6 whereas in Candida albicans, it is necessary for cell wall integrity and virulence.Citation7
The first Arabidopsis thaliana mutant lacking complex N-glycans was reported in 1993.Citation8 Since then, several mutants and transgenic plants altered in N-glycan maturation in the Golgi apparatus have been reported.Citation9–Citation12 Plants with altered N-glycan modification pathways that are devoid of potentially immunogenic complex N-glycans are used for the production of pharmaceutical proteinsCitation12,Citation13 and could serve as potential food crops with reduced allergenicity. Until recently, however, plant complex N-glycans have not been associated with essential biological functions in their host plants due to lack of obvious phenotypes of mutant plants defective in complex N-glycan biosynthesis. We recently reported that mutants defective in complex N-glycans show enhanced salt sensitivity, establishing that complex N-glycans are indispensable for certain biological functions.Citation1
Our previous study using an OST subunit mutant stt3a indicated that protein glycosylation could affect salt tolerance and root growth of A. thaliana.Citation14 Since OST functions upstream of protein folding processes in the ER, stt3a caused an unfolded protein response (UPR), which is a general ER stress response to protein folding defects, as well as accumulation of under-glycosylated proteins. In our recent study, we tried to address whether the salt stress response of the mutant is caused by an activation of UPR, or by a shortage of functional glycoproteins produced by the cells.Citation1 The cgl1 mutant is defective in N-acetylglucosaminyltransferase in the Golgi apparatusCitation15 and only able to produce oligomannosidic-type N-glycans but not complex-type N-glycans.Citation8 cgl1 mutants exposed to salt stress exhibited root growth arrest and radial swelling similar to stt3a mutants, however, unlike stt3a, the cgl1 mutation did not cause UPR as judged by expression of an UPR marker gene, BiPpro-GUS. This indicated that salt sensitivity of cgl1 (and likely also of stt3a) is due to lack of mature N-glycans essential for functionality of certain glycoprotein(s).
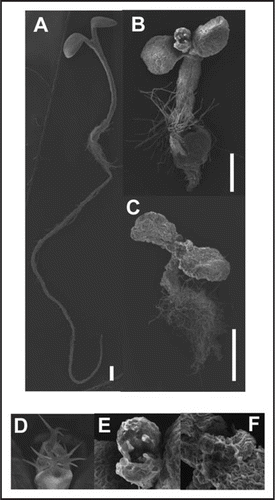
We have determined that a membrane-anchored β1,4-endoglucanase, KORRIGAN1/RADIAL SWELLING2 (KOR1/RSW2), which functions in cellulose biosynthesis, is a target of CGL1 and involved in the salt stress response of A. thaliana.Citation1 A temperature sensitive rsw2-1 alleleCitation16 showed specific genetic interaction with both cgl1 and stt3a mutations. The corresponding double mutants exhibited spontaneous growth defects at the permissive temperature that were reminiscent of those of rsw2-1 at the restrictive temperature, of cgl1 and stt3a plants treated with salt, and of the rsw1-1 rsw2-1 double mutant that combines two cellulose deficiency mutations. This showed that cgl1 and stt3a enhance cellulose deficiency of rsw2-1, and in turn indicate that the KOR1/RSW2 protein requires complex N-glycans for its function in vivo. Further pyramiding of these mutations resulted in incremental enhancement of growth defects as well as developmental defects of the host plants (Kang et al., (2008), and Fig. 1). Importance of functional cellulose biosynthesis for salt tolerance was further supported by the novel finding of increased salt-sensitivity of rsw2-1 and rsw1-1 single mutants.Citation1
Our previous and current data have implications that affect our view of protein N-glycosylation in plants. First, after all, plant complex N-glycans confer important in vivo functions to secreted/secretory glycoproteins, i.e., protect root growth from salt/osmotic stress. In contrast to core oligosaccharides in the ER, which globally affect protein folding, complex N-glycans appear to function at the individual protein level. Second, one of the targets of salt/osmotic stress is a component of the cellulose biosynthesis machinery, namely KOR1/RSW2 that requires complex N-glycans for its function. KOR1/RSW2 provides a link to how complex N-glycans protect plants from salt/osmotic stress. However, the mechanism by which salt stress triggers the growth arrest via KOR1/RSW2 dysfunction is not yet understood. We have previously shown that the root apical meristem of stt3a exhibits cell cycle arrest under salt stress, but cell differentiation and lateral root formation continued in the same root tip.Citation14 This implies that plants, in response to salt stress and compromised cell-wall biosynthesis at the root apical meristem, specifically attenuate cell cycle progression at the old meristem and initiate new meristems. A signal transduction pathway that coordinates cell-wall integrity and cell proliferation is well documented in Sacchromyces cerevisiae, where Protein kinase C1 (Pkc1) and a MAP kinase cascade play essential roles.17 Interestingly, both S. cerevisiae Stt3 and Och1 (a mannosyltransferase in the Golgi apparatus) are involved in the cell-wall integrity pathway.Citation17 In A. thaliana, mutations in the receptor kinase THESEUS1 suppressed hypocotyl elongation defects and ectopic lignification in several cellulose deficient mutants.Citation18 However, since THE1 is expressed in elongation zones but not in cell division zones of root tips, and the1 did not suppress the kor1-1 phenotype,Citation18 it is unlikely that THE1 is involved in the regulation of the salt stress response at the root apical meristem. This implies that dividing cells and expanding cells employ distinct mechanism to sense cellulose deficiency. Understanding how complex N-glycans regulate cell-wall biosynthesis and cell proliferation is an exciting task for the coming years.
Figures and Tables
Figure 1 Scanning electron micrograph of one-week-old wild type (A and D), rsw2-1 stt3a-2 cgl1-T (B and E) and rsw2-1 rsw1-1 stt3a-2 cgl1-T (C and F) seedlings grown at 18°C. Severe growth defects in mutants are obvious. In shoot apical meristem (D–F), aberrant trichome development is seen in rsw2-1 stt3a-2 cgl1-T (E). In rsw2-1 rsw1-1 stt3a-2 cgl1-T (F), the meristem is transformed into unorganized mass of cells. Bars indicate 0.5 mm.
Addendum to:
References
- Kang JS, Frank J, Kang CH, Kajiura H, Vikram M, Ueda A, Kim S, Bahk JD, Triplett B, Fujiyama K, Lee SY, von Schaewen A, Koiwa H. Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc Natl Acad Sci USA 2008; 105:5933 - 5938
- Kukuruzinska MA, Lennon K. Protein N-glycosylation: molecular genetics and functional significance. Crit Rev Oral Biol Med 1998; 9:415 - 448
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science 2001; 291:2364 - 2369
- Lerouge P, Cabanes Macheteau M, Rayon C, Fischette Lainé AC, Gomord V, Faye L. N-Glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol Biol 1998; 38:31 - 48
- Freeze HH. Human disorders in N-glycosylation and animal models. Biochim Biophys Acta 2002; 1573:388 - 393
- Sarkar M, Leventis PA, Silvescu CI, Reinhold VN, Schachter H, Boulianne GL. Null mutations in Drosophila N-acetylglucosaminyltransferase I produce defects in locomotion and a reduced life span. J Biol Chem 2006; 281:12776 - 12785
- Bates S, Hughes HB, Munro CA, Thomas WP, MacCallum DM, Bertram G, Atrih A, Ferguson MA, Brown AJ, Odds FC, Gow NA. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem 2006; 281:90 - 98
- von Schaewen A, Sturm A, O'Neill J, Chrispeels M. Isolation of a mutant Arabidopsis plant that lacks N-acetyl glucosaminyl transferase I and is unable to synthesize Golgi-modified complex N-linked glycans. Plant Physiol 1993; 102:1109 - 1118
- Strasser R, Schoberer J, Jin C, Glossl J, Mach L, Steinkellner H. Molecular cloning and characterization of Arabidopsis thaliana Golgi alpha-mannosidase II, a key enzyme in the formation of complex N-glycans in plants. Plant J 2006; 45:789 - 803
- Strasser R, Altmann F, Mach L, Glossl J, Steinkellner H. Generation of Arabidopsis thaliana plants with complex N-glycans lacking beta1,2-linked xylose and core alpha1,3-linked fucose. FEBS Lett 2004; 561:132 - 136
- Fitchette-Laine AC, Gomord V, Cabanes M, Michalski JC, Saint Macary M, Foucher B, Cavelier B, Hawes C, Lerouge P, Faye L. N-glycans harboring the Lewis a epitope are expressed at the surface of plant cells. Plant J 1997; 12:1411 - 1417
- Strasser R, Stadlmann J, Schahs M, Stiegler G, Quendler H, Mach L, Glossl J, Weterings K, Pabst M, Steinkellner H. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J 2008; 6:392 - 402
- Koprivova A, Stemmer C, Altmann F, Hoffmann A, Kopriva S, Gorr G, Reski R, Decker EL. Targeted knockouts of Physcomitrella lacking plant-specific immunogenic N-glycans. Plant Biotechnol J 2004; 2:517 - 523
- Koiwa H, Li F, McCully MG, Mendoza I, Koizumi N, Manabe Y, Nakagawa Y, Zhu JH, Rus A, Pardo JM, Bressan RA, Hasegawa PM. The STT3a subunit isoform of the Arabidopsis thaliana oligosaccharyltransferase controls adaptive responses to salt/osmotic stress. Plant Cell 2003; 15:2273 - 2284
- Saint-Jore-Dupas C, Nebenfuhr A, Boulaflous A, Follet Gueye ML, Plasson C, Hawes C, Driouich A, Faye L, Gomord V. Plant N-glycan processing enzymes employ different targeting mechanisms for their spatial arrangement along the secretory pathway. Plant Cell 2006; 18:3182 - 3200
- Lane DR, Wiedemeier A, Peng L, Hofte H, Vernhettes S, Desprez T, Hocart CH, Birch RJ, Baskin TI, Burn JE, Arioli T, Betzner AS, Williamson RE. Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-beta-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol 2001; 126:278 - 288
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 2005; 69:262 - 291
- Hematy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Hofte H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 2007; 17:922 - 931