Abstract
Nectar properties (volume, concentration, viscosity) change dynamically in time. As stated by Pedersen some decades ago (1958), “Nectar is not a static product remaining outside the plant once produced but is in close contact with the plant system.”1 It is now evident that secretion may occur concomitantly with resorption and that the latter process sometimes continues after secretion has ended. The rate of the two processes may be modified dynamically by the plant in response to ecological and physiological constraints, maintaining a relatively constant nectar concentration to ensure pollinator visits (nectar homeostasis) and reallocating resources, especially during development of the ovules and pericarp after fertilization. We suspect that nectar resorption is under-estimated as a phenomenon, because it requires detailed information on the dynamics of nectar production throughout the life of the flower that is seldom available or taken into consideration. The cytological and molecular mechanisms involved in nectar resorption are almost completely unknown. Sugar sensing may have a fundamental role in nectar resorption and homeostasis. Due to direct contact with sugar solutions, nectaries may offer wide scope for insights into this phenomenon which has attracted interest as part of plant signalling systems.
Addendum to: Nepi M, Stpiczyńska M. The complexity of nectar: Secretion and resorption dynamically regulate nectar features. Naturwissenschaften 2008 95:177-84.
Reasons of Nectar Resorption
Two main functions can be recognized for nectar resorption: the recovery of resources invested in nectar production and a homeostatic mechanism during nectar secretion and presentation.
Nectar production requires considerable expenditure of energyCitation2 and as estimated, the energy invested in nectar production by Medicago sativa can be twice the energy invested in seeds.Citation3 Recovery of resources is therefore an important advantage for plants that reutilise this source of carbohydrates in uncollected nectar. In Cucurbita pepo and Platanthera chlorantha, all unconsumed nectar is reclaimed, maximising the recovery of energy invested in nectar production.Citation4
Nectar resorption also plays an important ecological function because it can be involved in nectar homeostatic mechanisms that enable regulation of nectar volume, concentration and thus viscosity by reducing the effect of water loss due to evaporation. Since nectar composition and concentration are adapted to the preferences of visitors, reduction of nectar viscosity by sugars resorption may facilitate nectar probing and this mechanism may be important to promote visits by efficient pollinators.
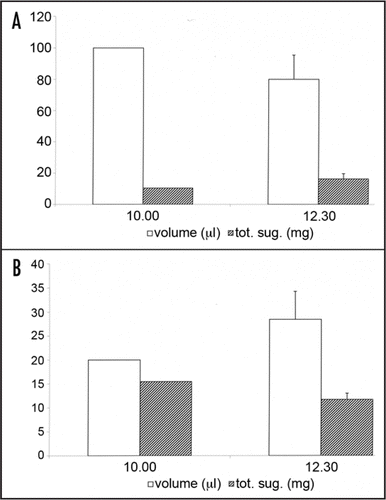
The two functions recognized for nectar resorption may be not mutually exclusive in a given species. It has been demonstrated already that in Cucurbita pepo, nectar resorption has the resources recovery function after flower closure.Citation5,Citation6 Moreover, experiments indicated the homeostatic function of nectar resorption during anthesis in squash. In this species, nectar secretion begins early in the morning, about 90 min before the flower opens (5.30–6 am, ), reaching a maximum (70 µl) just after flower opening. It subsequently decreases, ceasing at about 9.30 am. Sugar concentration is not constant during this period but follows the pattern of nectar volume, reaching a maximum (43% w/w) just after the beginning of anthesis, followed by a slight decrease. There is no apparent production from 9.30 am to the end of anthesis (about 12 noon). To determine whether the plant can modify nectar dynamically, we performed nectar substitution experiments. At 10.30 am the nectar was removed from 16 male flowers and substituted with 100 µl of diluted nectar (10% w/w) or 20 µl of concentrated nectar (60% w/w). After two hours (12.30 noon) we sampled the same flowers and measured nectar volume and concentration. The results are shown in . The volume of diluted nectar decreased and its concentration increased so much that the total sugar content rose (). On the contrary, the volume of concentrated nectar increased and its concentration decreased, resulting in a decrease in total sugar (). In the case of diluted nectar there is a net efflux of sugars and a net influx of water in the nectary while the opposite happens in the case of concentrated nectar. These results demonstrated clearly that the plant can dynamically modify nectar concentration after its secretion. This suggests the existence of a sugar sensing mechanism that allows a certain degree of nectar homeostasis. As a consequence of this mechanism, both the water and sugar component can be secreted or reabsorbed according to whether the nectar on the nectary surface is diluted or concentrated. It can be concluded that nectary can separately regulate nectar concentration and volume.
Sugar Sensing Mechanism
Although there is relatively good evidence that nectar resorption occurs, there are few detailed studies on transport and incorporation of resorbed sugars. A question that needs to be addressed is how nectar is resorbed at the nectary cell level. First of all, nectaries are supposed to have a “sugar sensing” mechanism for regulating the volume and concentration of nectar. Resorption from high nectar volume was demonstrated by Nicolson in Grevillea robustaCitation7 and she hypothesized that resorption took place when nectar reached a threshold volume and came in contact with a presumptive “reabsorptive” area. Castellanos et al., also assumed receptors located at an appropriate place in flowers to allow regulation of nectar “level” in the manner of a float valve.Citation8
Sugar secretion may occur passively on the basis of a concentration gradient, whereas regulation of concentration in the apoplast may be achieved by sucrose hydrolysis and/or sugar resorption. Perhaps resorption could be a response to modifications of cell turgor which in turn responds rapidly to changes in osmolality (Castellanos et al.,Citation8 and references therein). Recent studies clarified the mechanisms of sugar sensing and the role of sugars as signalling molecules in plant metabolism.Citation9–Citation13 Although the regulatory effect of sugar on photosynthetic activity and plant metabolism has long been recognized, the concept of sugar as central signalling molecules is relatively novel.Citation12 It is now activities in source (sugar producing) and sink (evident that plant use sugars as metabolic messengers or signalling molecules to coordinate metabolic sugar consuming) during plant growth and development. Sugar signalling also interact with hormone, light and stress signalling constituting a signalling networks in plants.Citation13 In relation to resource allocation, the ability to sense altered sugar concentrations could have important advantages by allowing the plant to adjust its metabolism in source tissues to meet the demand in sinks. The quality and quantity of sugars accumulated in the plant cell can modulate mitotic activity and cell development.Citation9 In the flower, sugars from reabsorbed nectar could be an indirect factor influencing on fruit and ovule development. Based on sugar signal transduction process in Saccharomyces, it has been proposed that members of the sugar transporter family in plants might play a role in sugar sensingCitation14 and a central role is recognized for hexokinase and sucrose transporters.Citation11–Citation13 In yeast, it has been shown that membrane-bound receptors closely related to sugar transporters are used to sense external sugar concentration and that are able to respond to a rapidly changing external environment regulating the transporter gene expression and insertion or degradation of proteins at the plasma membrane.Citation10 Since it was demonstrated that the same class of proteins are involved in sugar sensing and transduction in plants too,Citation11 and that there is an in vivo response to the different amount of soluble sugars in plant tissues,Citation15 it is reasonable to hypothesise such a mechanism to operate in nectaries. In this perspective, the processes of nectar secretion and resorption could be interpreted not as two distinct processes but as two aspects of a whole phenomenon that is modulated according the external environment (higher or lower nectar concentration) and internal source-sink relationships.
Figures and Tables
Figure 1 Nectar production in Cucurbita pepo male flowers. The flowers open at 5.30 a.m. A maximum of both nectar volume and concentration was reached at 6.30 a.m. There is no apparent nectar secretion from 10.30 to the end of anthesis (12.00).
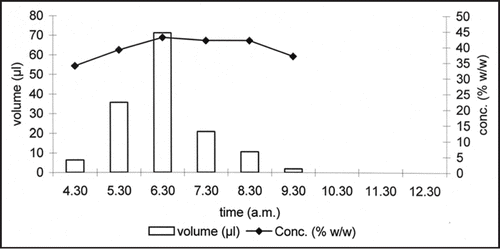
Figure 2 Volume, concentration and total sugar changes in the nectar substitution experiments of Cucurbita pepo. Large amount of diluted nectar (A) stimulated nectary to reabsorb water and add sugars while small amount of concentrated nectar (B) stimulated nectary to reabsorb sugars and add water. Bars = s.d.; n = 8.
Addendum to:
References
- Pedersen MW, LeFevre CW, Wiebe HH. Absorption of C14-labelled sucrose by alfalfa nectaries. Science 1958; 127:758 - 759
- Pyke GH. What does it cost a plant to produce nectar?. Nature 1991; 350:58 - 59
- Southwick EE. Photosynthate allocation to floral nectar: a neglected energy investment. Ecology 1984; 65:1775 - 1779
- Nepi M, Stpiczynska M. Nectar resorption and translocation in Cucurbita pepo L. and Platanthera chlorantha Custer (Rchb.). Plant Biol 2007; 9:93 - 100
- Nepi M, Ciampolini F, Pacini E. Development and ultrastructure of Cucurbita pepo nectaries of male flowers. Ann Bot 1996a; 78:95 - 104
- Nepi M, Pacini E, Willemse MTM. Nectary biology of Cucurbita pepo: ecophysiological aspects. Acta Bot Neerl 1996b; 45:41 - 54
- Nicolson SW. Direct demonstration of nectar resorption in the flowers of Grevillea robusta (Proteaceae). Funct Ecol 1995; 9:584 - 588
- Castellanos MC, Wilson P, Thomson JD. Dynamic nectar replenishment in flowers of Penstemon (Scrophulariaceae). Am J Bot 2002; 89:111 - 118
- Borisijuk L, Walenta S, Weber H, Mueller Kieser W, Wobus U. High resolution histographical mapping of glucose concentrations in developing cotyledons of Vicia faba in relation to mitotic activity and storage processes: glucose as a possible developmental trigger. Plant J 1998; 154:583 - 591
- Williams L, Lemoine R, Sauer N. Sugar transporters in higher plants—a diversity of roles and complex regulation. Trends Plant Sci 2000; 5:283 - 290
- Loreti E, De Bellis L, Alpi A, Perata P. Why and how do plant cells sense sugars?. Ann Bot 2001; 88:803 - 812
- Rolland F, Moore B, Sheen J. Sugar sensing and signalling in plants. Plant Cell 2002; Supplement:185 - 205
- Rolland F, Sheen J. Sugar sensing and signalling networks in plants. Biochem Soc Transact 2005; 33:269 - 271
- Lalonde S, Boles B, Hellmann H, Barker H, Patrick JF, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell 1999; 11:707 - 726
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidences for in vivo sugar sensing in Arabidopsis. J Plant Res 2006; 119:115 - 123