Abstract
Cysteine biosynthesis in plants takes place in the three cellular compartments with autonomous protein biosynthesis machinery: cytosol, plastids and mitochondria. This sulfur-containing molecule is synthesized sequentially in these compartments by two enzymatic families, the serine acetyltransferases and the O-acetylserine(thiol)lyases. Each family consists of several isoforms that differ in subcellular localization and abundance. Why so many isoforms are required in plant cell for cysteine biosynthesis has remained unknown to date. The characterization of gene-specific knockout mutants has started to address this question. In our recent work, we have performed a detailed analysis of the Arabidopsis oas-a1 null mutant and showed that the antioxidant capacity of the cytosol is compromised highlighting the contribution of cytosolic Cys in redox signaling.
Addendum to: Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in Arabidopsis. López-Martín MC, Becana M, Romero LC, Gotor C. Plant Physiol 2008; In press.
Sulfur is a macronutrient essential for plant growth and development and constitutes the 0.3–0.5% of the total dried weight. Sulfur is very important for plants because is found in the amino acids cysteine (Cys) and methionine, and in many other cellular components as glutathione (GSH). Glutathione is the major non-protein thiol in plant tissues and is regarded as one of the major determinants of cellular redox homeostasis. Its roles include acting as a mobile pool of reduced sulfur, involvement in the detoxification of xenobiotics, protection against heavy metal toxicity, source of reductant in enzymatic reactions, effects on growth and development, regulation of gene expression, resistance to pathogen infection and tolerance to environmental perturbations that promote oxidative stress.Citation1 The sulfur moiety of the majority of the plant sulfur-compounds including GSH is derived from Cys, which is the final product of the primary sulfate assimilation pathway. Therefore, their biosynthetic pathways are intimately linked.
The biosynthesis of Cys is accomplished by the sequential reaction of two enzymes, serine acetyltransferase (SAT) that synthesizes the intermediary product O-acetylserine (OAS) from acetyl-CoA and serine; and O-acetylserine(thiol)lyase (OASTL) that incorporates the sulfide coming from the assimilatory reduction of sulfate to OAS producing Cys. Together both enzymes form the hetero-oligomeric Cys synthase complex described for the first time in bacteria and extensively studied in plants later on. The protein interactions within the complex strongly modify the kinetic properties of SAT, the enzyme becoming more efficient for OAS synthesis. OASTL by contrast is active in its abundant free form but exhibits much reduced activity when complexed with SAT.Citation2
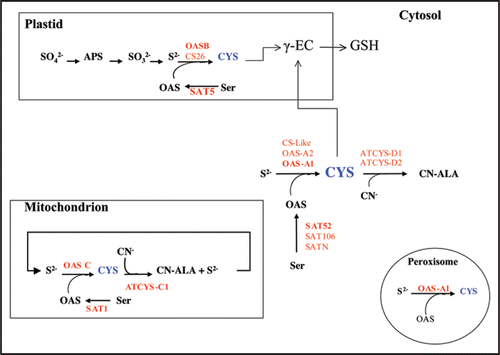
The plant cells contain different SAT and OASTL enzymes localized in the cytosol, plastid and mitochondrion, resulting in a complex variety of isoforms and in different subcellular Cys pools (). Arabidopsis thaliana is the best investigated plant and in its genome five different SATCitation3 and nine OASTL genesCitation4 have been identified. The presence of multiple SAT and OASTL cDNAs in the databases suggests the organization to be similar in other plant species. Thus, the information provided by the TIGR Rice Genome Annotation database (http://rice.tigr.org) allows to identify six SAT and eleven OASTL genes in the rice (Oryza sativa subsp. japonica cv. Nipponbare) genome.
In Arabidopsis, the most abundant OASTL genes at the transcriptional level encode the cytosolic OAS-A1, the plastidial OASB and the mitochondrial OASC isoforms. One of the genes, OAS-A2, does not produce functional protein due to an in-frame stop codon and an unspliced intron. Null alleles of the oasA1 and oasB showed that the major cytosolic and plastidial enzymes are dispensable for growth under normal conditions, although together they contribute to 95% of total OASTL activity.Citation5 The genes coding for CS26 and CS-like are transcribed to low level and their functional roles have not been explored. The other three isoforms, CYS-C1, CYS-D1 and CYS-D2 are in fact β-cyanoalanine synthase enzymes that use Cys to catalyze the detoxification of cyanide.
The work of Heeg and co., demonstrates that Cys and sulfide are exchangeable between the cystosol and the organelles. The fact that plant growth is not significantly affected in single null OASTL mutants suggests there is a partial functional redundancy between the major isoforms to supply Cys for protein synthesis and S-reduced needed for growth. However, why plants require so many OASTL isoforms and why Cys biosynthesis occurs in the three cellular compartments remain unknown.
In our recent work, we have addressed these questions and highlighted some clues about the contribution of cytosolic Cys in redox signaling and ROS detoxification. We have performed a detailed analysis of the oasA1 null mutant and showed that the antioxidant capacity of the cytosol is compromised and that an elevated accumulation of hydrogen peroxide is detected. In addition, we have observed in the knockout mutant a significant shift of the glutathione redox state in favour of its oxidized form suggesting the importance of cytosolic Cys pool in maintenance of the cellular redox state. Interestingly, a recent proteome analysis of Arabidopsis leaf peroxisomes has revealed the presence of the cytosolic OAS-A1 isoform within this compartment which is involved in primarily oxidative metabolic reactions.Citation6 Since enzymes involved in glutathione biosynthesis are absent from the peroxisome, the Cys pool produced by the OAS-A1 isoform in the cytosol and the peroxisome has a redox regulation or signaling function rather than a biosynthetic purpose. In fact, there are an increasing number of reports from animal cells showing that the plasma GSH/GSSG ratio is not in equilibrium with the plasma Cys/cystine pool. For instance, Cys availability and the Cys/cystine redox couple regulate the p44/p42 mitogen-activated protein kinase (MAPK) pathway and cell proliferation in intestinal cells and this regulation occurs without altering the intracellular GSH redox potential.Citation7 The discrepancy between the GSH redox status of the Arabidopsis mutant rax1-1,Citation8 and the oas-a1 null mutant and their respective contents in Cys and GSH suggest that compartmental cytosolic/peroxisome Cys may serve as an independent node for redox signalling and control in plants.
In conclusion, although major plant OASTL isoforms can be redundant under normal growth conditions and Cys can be translocated from the organelles to the cytosol and vice versa, each compartmental Cys pool and their biosynthesis should be crucial under transient stress situation in the plant as a consequence of environmental changes.
Figures and Tables
Figure 1 Biosynthesis of cysteine and subcellular localization of SAT and OASTL isoforms in Arabidopsis thaliana. Primary sulfur assimilation and reduction takes place in plastids where is reduced to sulfide. The sulfide is incorporated to the carbon skeleton of OAS within the plastid, or diffuses to the cytosol and the mitochondria, to form the cysteine molecule. For each compartment, the SAT and OASTL isoforms involved in the catalysis are shown (bold red font represent the major isoform). The β-cyanoalanine synthase isoforms are also shown in the diagram.
Acknowledgements
This work was funded by Ministerio de Educación y Ciencia (grant no. BIO2007-62770) and Junta de Andalucía (grant no. CVI-273), Spain.
Addendum to:
References
- Meyer AJ, Hell R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res 2005; 86:435 - 457
- Droux M, Ruffet ML, Douce R, Job D. Interactions between serine acetyltransferase and O-acetylserine(thiol)lyase in higher plants—Structural and kinetic properties of the free and bound enzymes. Eur J Biochem 1998; 255:235 - 245
- Howarth JR, Domínguez-Solís JR, Gutiérrez-Alcalá G, Wray JL, Romero LC, Gotor C. The serine acetyltransferase gene family in Arabidopsis thaliana and the regulation of its expression by cadmium. Plant Mol Biol 2003; 51:589 - 598
- Wirtz M, Droux M, Hell R. O-acetylserine(thiol)lyase: an enigmatic enzyme of plant cysteine biosynthesis revisited in Arabidopsis thaliana. J Exp Bot 2004; 55:1785 - 1798
- Heeg C, Kruse C, Jost R, Gutensohn M, Ruppert T, Wirtz M, Hell R. Analysis of the Arabidopsis O-Acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell 2008; 20:168 - 185
- Reumann S, Babujee L, Ma CL, Wienkoop S, Siemsen T, Antonicelli GE, Rasche N, Luder F, Weckwerth W, Jahn O. Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways and defense mechanisms. Plant Cell 2007; 19:3170 - 3193
- Nkabyo YS, Go YM, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol-Gastroint Liver Physiol 2005; 289:70 - 78
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, Karpinski S, Mullineaux PM. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 2004; 16:2448 - 2462