Abstract
The significance of cell wall invertase (cwINV) for plant defense was investigated by comparing wild type (wt) tobacco Nicotiana tabacum L. Samsun NN (SNN) with plants with RNA interference-mediated repression of cwINV (SNN::cwINV) during the interaction with the oomycetic phytopathogen Phytophthora nicotianae. We have previously shown that the transgenic plants developed normally under standard growth conditions, but exhibited weaker defense reactions in infected source leaves and were less tolerant to the pathogen. Here, we show that repression of cwINV was not accompanied by any compensatory activities of intracellular sucrose-cleaving enzymes such as vacuolar and alkaline/neutral invertases or sucrose synthase (SUSY), neither in uninfected controls nor during infection. In wt source leaves vacuolar invertase did not respond to infection, and the activity of alkaline/neutral invertases increased only slightly. SUSY however, was distinctly stimulated, in parallel to enhanced cwINV. In SNN::cwINV SUSY-activation was largely repressed upon infection. SUSY may serve to allocate sucrose into callose deposition and other carbohydrate-consuming defense reactions. Its activity, however, seems to be directly affected by cwINV and the related reflux of carbohydrates from the apoplast into the mesophyll cells.
Addendum to: Essmann J, Schmitz-Thom I, Schön H, Sonnewald S, Weis E, Scharte J. RNAi-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol 2008; 10.1104/pp.108.121418
Plant defense against pathogens is costly in terms of energy and carbohydrates.Citation1,Citation2 Sucrose (Suc) and its cleavage products glucose and fructose are central molecules for metabolism and sensing in higher plants (reviewed in refs. Citation3 and Citation4). Rapid mobilization of these carbohydrates seems to be an important factor determining the outcome of plant-pathogen interactions. In particular in source cells reprogramming of the carbon flow from Suc to hexoses may be a crucial process during defense.Citation1,Citation2
There are two alternative routes of sucrolytic carbohydrate mobilization. One route is reversible and involves an uridine 5′-diphosphate (UDP)-dependent cleavage catalyzed by sucrose synthase (SUSY). Its activity is limited by the concentrations of Suc and UDP in the cytosol, as the affinity of the enzyme to its substrate is relatively low (Km for Suc 40–200 mM). The other route is the irreversible, hydrolytic cleavage by invertases (INVs), which exhibit high affinity to Suc (Km 7–15 mM).Citation5
Plants possess three different types of INV isoenzymes, which can be distinguished by their solubility, subcellular localization, pH-optima and isoelectric point. Usually, they are subdivided into cell wall (cwINV), vacuolar (vacINV), and alkaline/neutral (a/nINVs) INVs.
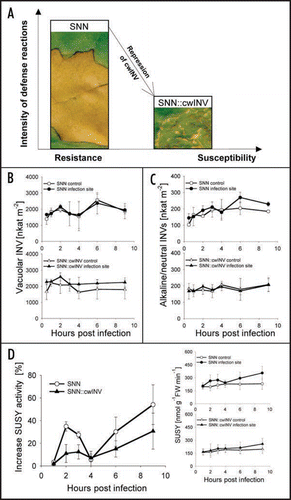
cwINV, also referred to as extracellular or apoplastic INV, is characterized by a low pH-optimum (pH 3.5–5.0) and usually ionically bound to the cell wall. It is the key enzyme of the apoplastic phloem unloading pathway and plays a crucial role in the regulation of source/sink relations (reviewed in refs. Citation3, Citation6–Citation8). A specific role during plant defense has been suggested, based on observations that cwINV is often induced during various plant-pathogen interactions, and the finding that overexpression of a yeast INV in the apoplast increases plant resistance.Citation6,Citation8–Citation10 It was shown, that a rapid induction of cwINV is, indeed, one of the early defense-related reactions in resistant tobacco source leaves after infection with Phytophthora nicotianae (P. nicotianae).Citation11 Finally, the whole infection area in wt leaves was covered with hypersensitive lesions, indicating that all cells had undergone hypersensitive cell death ().Citation1,Citation11 When the activity of cwINV was repressed by an RNAi construct, defense-related processes were impaired, and the infection site exhibited only small spots of hypersensitive lesions. Finally, the pathogen was able to sporulate, indicating a reduced resistance of these transgenic plants ().Citation1
vacINV, also labeled as soluble acidic INV, is characterized by a pH optimum between pH 5.0–5.5. Among others it determines the level of Suc stored in the vacuole and generates hexose-based sugar signals (reviewed in refs. Citation3 and Citation12). Yet, no specific role of vacINV during pathogen response has been reported. Although vacINV and cwINV are glycoproteins with similar enzymatic and biochemical properties and share a high degree of overall sequence homology and two conserved amino acid motifs,Citation4 the activity of vacINV in tobacco source leaves was not changed due to the repression of the cwINV ().Citation1 After infection with P. nicotianae the activity of vacINV in wt SNN did not respond under conditions where cwINV was stimulated.Citation1 There was also no significant change in the transgenic SNN::cwINV (). This suggests that during biotic stress, there is no crosstalk between the regulation of cwINV and vacINV.
a/nINVs exhibit activity maxima between pH 6.5 and 8.0, are not glycosylated and thought to be exclusively localized in the cytosol. But recent reports also point to a subcellular location in mitochondria and chloroplasts.Citation13,Citation14 Only a few a/nINVs have been cloned and characterized, and not much is known about their physiological functions (reviewed in refs. Citation4, Citation14 and Citation15). Among other things they seem to be involved in osmotic or low-temperature stress response.Citation14,Citation15 During the interaction between tobacco and P. nicotianae the activity of a/nINVs rose on average 17% in the resistant wt SNN between 1 to 9 hours post infection (). By contrast, in SNN::cwINV the a/nINVs activities remained unchanged in control leaves and even after infection (). This suggests that the defense related stimulation in a/nINVs activities is rather a secondary phenomenon, possibly in response to the enhanced cwINV activity and the related carbohydrate availability in the cytosol.
SUSY can be found as a soluble enzyme in the cytosol, bound to the inner side of the plasma membrane or the outer membrane of mitochondria, depending on the phosphorylation status. It channels hexoses into polysaccharide biosynthesis (i.e., starch, cellulose and callose) and respiration.Citation12,Citation16 There is also evidence that SUSY improves the metabolic performance at low internal oxygen levelsCitation17 but little is known about its role during plant defense. Callose formation is presumably one of the strongest sink reactions in plant cells.Citation1,Citation18 Defense-related SUSY activity may serve to allocate Suc into callose deposition and other carbohydrate-consuming defense reactions. In fact, in the resistant wt the activity of SUSY increased upon interaction with P. nicotianae in a biphasic manner (). The time course is comparable to that of cwINV activity and correlates with callose deposition and enhanced respiration.Citation1,Citation11 However, repression of cwINV leads in general to a reduction of SUSY activity in source leaves of tobacco.Citation1 After infection the activation of SUSY was also significantly impaired (). At the same time, the early defense-related callose deposition in infected mesophyll cells of SNN::cwINV plants is substantially delayed.Citation1 It is known that expression of SUSY isoforms is differentially controlled by sugars,Citation12 and there is evidence that hexoses generated by the defense-induced cwINV activity deliver sugar signals to the infected cells.Citation1 In this sense, the reduction of defense-related, cwINV-generated sugar signals could be responsible for the repression of SUSY activity in SNN::cwINV plants after infection with P. nicotianae.
Only limited hexoses or hexose-based sugar signals could be generated by cytoplasmic Suc cleavage.Citation12 The reduction of soluble carbohydrates for sugar signaling and also as fuel for metabolic pathways that support defense reactions could be responsible for the impaired resistance in SNN::cwINV plants ().
Obviously, neither intracellular INV isoforms, nor SUSY can compensate for the reduced carbohydrate availability due to cwINV repression during plant defense. The data also suggest that the activity of SUSY is affected by cwINV and related reflux of carbohydrates. It is known that SUSY activity can be controlled, e.g., by sugar-mediated phosphorylationCitation12 and one may speculate that posttranslational modulation of the protein is affected by the defense-related carbohydrate status of the cell.
Abbreviations
| INV | = | invertase |
| cwINV | = | cell wall invertase |
| vacINV | = | vacuolar invertase |
| a/nINVs | = | alkaline/neutral invertases |
| SUSY | = | sucrose synthase |
| UDP | = | uridine 5′-diphosphate |
| wt | = | wild type |
Figures and Tables
Figure 1 Defense-induced changes in the activity of intracellular sucrose-cleaving enzymes and their contribution to defense. (A) The repression of cwINV in source leaves of tobacco leads to impaired pathogen resistance and can not be compensated by other sucrose-cleaving enzymes. The intensity of defense reactions is amongst others indicated by the extent of hypersensitive lesions. (B and C) Absolute activity of vacuolar (B) and alkaline/neutral (C) INVs at the infection site (white symbols, control; black symbols, infection site). (D) Increase in SUSY activity at the infection site. All data points taken from noninfected control parts of the plants in each individual experiment and each point along the time scale of an experiment are set as 0%. At least three independent infections are averaged and their means are presented as percentage changes ± SE (circles, SNN; triangles, SNN::cwINV). Insets show the means of the absolute amount of activities (white symbols, control; black symbols, infection site). Material and methods according to Essmann, et al.Citation1
Acknowledgements
We are grateful to Sophia Sonnewald (FAU, Erlangen, Germany) for providing the SNN::cwINV plants and Yves Gibon (MPI of Molecular Plant Physiology, Golm, Germany) for helpful assistance with SUSY measurements.
Addendum to:
References
- Essmann J, Schmitz-Thom I, Schon H, Sonnewald S, Weis E, Scharte J. RNAi-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol 2008; 47:1288 - 1299
- Berger S, Sinha AK, Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant pathogen interactions. J Exp Bot 2007; 58:4019 - 4026
- Roitsch T, González MC. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 2004; 9:606 - 613
- Rolland F, González EB, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann Rev Plant Biol 2006; 57:676 - 709
- Avigad G. Loewus TA, Tanner W. Sucrose and other disaccharides. Encyclopedia of Plant Physiology 1982; Heidelberg, Germany Springer-Verlag 217 - 347
- Ehness R, Ecker M, Godt D, Roitsch T. Glucose and stress independently regulate source/sink relations and defence mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 1997; 9:1825 - 1841
- Roitsch T. Source-sink regulation by sugar and stress. Curr Opin Plant Biol 1999; 2:198 - 206
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK. Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 2003; 54:513 - 524
- Herbers K, Meuwly P, Frommer W, Metraux J, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 1996; 8:793 - 803
- Herbers K, Takahata Y, Melzer M, Mock HP, Hajirezaei M, Sonnewald U. Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol Plant Pathol 2000; 1:51 - 59
- Scharte J, Schön H, Weis E. Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae. Plant Cell Environ 2005; 28:1421 - 1435
- Koch K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 2004; 7:235 - 246
- Murayama H, Handa S. Genes for alkaline/neutral invertase in rice: alkaline/neutral invertases are located in plant mitochondria and also in plastids. Planta 2007; 225:1193 - 1203
- Vargas WA, Pontis HG, Salerno GL. New insights on sucrose metabolism: evidence for an active A/N-Inv in chloroplasts uncovers a novel component of the intracellular carbon trafficking. Planta 2008; 227:795 - 807
- Vargas WA, Pontis HG, Salerno GL. Differential expression of alkaline and neutral invertases in response to environmental stresses: characterization of an alkaline isoform as a stress-response enzyme in wheat leaves. Planta 2007; 226:1535 - 1545
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 1995; 92:9353 - 9357
- Bologa KL, Fernie AR, Leisse A, Loureiro ME, Geigenberger P. A bypass of sucrose synthase leads to low internal oxygen and impaired metabolic performance in growing potato tubers. Plant Physiol 2003; 132:2058 - 2072
- Maor R, Shirasu K. The arms race continues: battle strategies between plants and fungal pathogens. Curr Opin Microbiol 2005; 8:399 - 404