Abstract
The mutualistic interaction of plants with arbuscular mycorrhizal (AM) fungi is characterized by an exchange of nutrients. The plant provides sugars in the form of hexoses to the heterotrophic fungus in return for phosphate as well as nitrogen, water, and micronutrients. Plant sucrose-cleaving enzymes are predicted to play a crucial role in hexose mobilization as these enzymes appear to be absent in the fungal partner. Here, recent findings concerning the function of plant apoplastic invertases in the AM symbiosis are discussed. Plants with modulated enzyme activity in roots and leaves provide additional insight on the complexity of the regulation of the AM interaction by apoplastic invertases as mycorrhization could be reduced or stimulated depending on the level of invertase activity and its tissue-specific expression.
Arbuscular mycorrhizas (AMs) are the most common type of mycorrhizasCitation1 and have been found in roots of more than 80% of the terrestrial plant species. AMs are intimate and, in most cases, mutualistic associations of plant roots and fungi of the phylum Glomeromycota.Citation2 They are crucial in the ecology and physiology of terrestrial plants, supporting plants under biotic (e.g., pathogen infection) or abiotic stress (e.g., nutrient or water deficiency and heavy metal stress). AMs have in common at least one feature with all interactions between plants and biotrophic microorganisms: the competition for carbohydrates. In the mutualistic association of AM the plant and the heterotrophic fungal partner have to share the carbohydrates which are assimilated by the plants' source leaves and translocated as sucrose to the sink tissue. Previous work revealed that AM-fungi are using intraradical hexoses, mainly glucose, as their carbon source.Citation3–Citation5 To date no fungal sucrose-cleaving enzymes have been identified. After evaluating published data about AM-induced activity and/or transcript levels of plant sucrose-cleaving enzymes,Citation6–Citation11 we suggest distinct roles for the different types of plant enzymes involved in providing the carbohydrate supply during mycorrhization: (i) Cytosolic invertases and sucrose-synthases are favored to supply the increased energy demand of (colonized) root cells to fulfill their higher metabolite activity and structural changes; (ii) vacuolar invertases might, particularly in early stages, release stored sugars for both the plant and the fungus, whereas (iii) extracellular, apoplast-located invertases might directly deliver hexoses to the apoplastic fungal structures and increase the sink function of the AM root where carbohydrate demand is high for both partners (i.e., during high colonization).
In addition to their function as carbon and energy sources, sugars also serve as important signals in the plant, regulating developmental as well as stress- and defense-related processes.Citation12 Particularly the apoplastic invertases are reported to be pathogenesis-related (PR) proteins.Citation13 Thus, to avoid the activation of plant defense mechanisms in the mutualistic interaction, induction of these enzymes by the AM-fungus has to occur in a fine-tuned manner. Indeed, we could show that the induction of the apoplastic invertase LIN6, which is known to be inducible by a whole set of stress-related external stimuli,Citation14–Citation16 is tightly controlled upon AM. In response to colonization, promoter activation and transcript accumulation of LIN6 was relatively low (compared to wounding of the root) and occurred in a cell-specific manner (near fungal structures and in the root phloem).Citation11
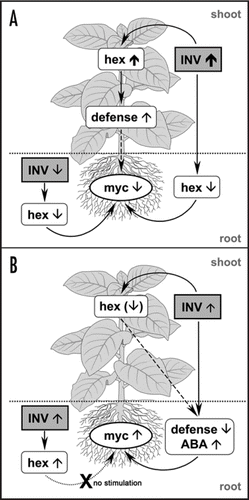
Interestingly, such a strictly controlled induction of apoplastic invertases is apparently sufficient for an optimal carbohydrate supply to the mycorrhizal root. Increased apoplastic invertase activity in the root accompanied by increased root hexose levels cannot improve mycorrhization.Citation17 There must be other mechanisms overtaking the regulatory control of the symbiont.Citation18,Citation19 In contrast, reduced apoplastic invertase activity in the root lowers root hexose levels and leads to a diminished mycorrhization, possibly by starvation effects on the fungus ().Citation17 This reveals the crucial impact of apoplastic invertases on the formation of an AM symbiosis.
Reduced mycorrhization is also exhibited by plants with drastically elevated levels of apoplastic invertase activity in the leaves.Citation20 The accumulation of hexoses in the source leaves does not only result in an undersupply of carbohydrates to the root, but also leads to an increased defense status.Citation20,Citation21 Even if transcripts for the analyzed PR proteins were only detected in the leaves, we can not exclude the possibility that some constitutive defense mechanisms have been activated which inhibit mycorrhization ().
There are limiting data, however, about a putative regulatory role for leaf apoplastic invertases on the root metabolism and/or biotic interactions of the root. However, there is recent evidence for a regulatory role of apoplastic invertases in leaves on the defense status of the root.Citation20 For the first time, we could demonstrate that slightly enhanced invertase activity in the leaf apoplast results in a strongly altered metabolite profile in the root, whereas less metabolic changes occurred in the leaves themselves. Some of the most significant metabolite changes observed in the root were reduced levels of amino acids, amines and phenolic compounds that are potentially involved in plant defense.Citation22–Citation24 Moreover, the abscisic acid content of the root increases, which can positively affect the susceptibility to biotrophic fungi, including AM fungi.Citation25 These metabolic changes in the root result in a stimulated mycorrhization which could not be achieved by simply increasing the hexose levels as described above (). Moreover, the steady-state levels of sugars in the roots of plants with slightly enhanced leaf invertase activity are not elevated (but are found to be slightly reduced), supporting a stronger regulatory role of plant secondary metabolism and/or hormone status than that of the pure nutritional benefit of the AM fungus. How such a regulation will also influence other biotic interactions remains to be elucidated.
In conclusion, we propose a more complex role for apoplastic invertases depending on their level of activity and tissue-specific expression in the plant: On the one hand, apoplastic invertases are efficiently regulating the carbon supply of the fungus by acting in the root. On the other hand, enhanced apoplastic invertase activity in the leaves can influence the defense status and the susceptibility of the root to AM fungi in two ways, leading either to a reduced or a stimulated colonization, depending on the level of enzyme activity.
Abbreviations
| AM | = | Arbuscular mycorrhiza |
| PR | = | pathogenesis related |
Figures and Tables
Figure 1 Regulation of arbuscular mycorrhization by modulated invertase activity in the plant apoplast. (A) A decreased mycorrhization can be achieved either by reduced activity of apoplastic invertase in the root or strongly increased activity in the leaf (up to 25-fold). In both cases, the root is characterized by an undersupply of carbohydrates. Moreover, the strong invertase activity in the source leaves results in sugar accumulation and activation of defense mechanisms in the leaves, that include the accumulation of PR transcript levels and phenolic compounds like chlorogenic acid isomers, scopolin and scopoletin.Citation22 This increased defense status of the plant may contribute additionally to the inhibition of AM. (B) An increased mycorrhization cannot, however, be obtained by increased invertase activity in the root apoplast despite the higher root hexose levels. Instead, stimulated mycorrhization was observed in plants with slightly (2- to 4-fold) enhanced invertase activity in the leaf apoplast. Such plants showed an altered defense and hormone status in the root as characterized by a reduction in phenolic compounds (chlorogenic acid, scopolin and scopoletin), amines and some amino acids as well as increased abscisic acid levels. These changes are potentially triggered by slightly reduced sugar levels in the leaves.
Addendum to:
References
- Smith SE, Read DJ. Mycorrhizal symbiosis. 2. Edition 1997; San Diego, USA Academic Press
- Schüssler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol Res 2001; 105:1413 - 1421
- Solaiman MZ, Saito M. Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol 1997; 136:533 - 538
- Pfeffer PE, Douds DD Jr, Bécard G, Shachar-Hill Y. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol 1999; 120:587 - 598
- Douds DD Jr, Pfeffer PE, Shachar-Hill Y. Application of in vitro methods to study carbon uptake and transport by AM fungi. Plant Soil 2000; 226:255 - 261
- Wright DP, Read DJ, Scholes JD. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ 1998; 21:881 - 891
- Blee KA, Anderson AJ. Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules. Plant Mol Biol 2002; 50:197 - 211
- Hohnjec N, Perlick AM, Pühler A, Küster H. The Medicago truncatula sucrose synthase gene MtSucS1 is activated both in the infected region of root nodules and in the cortex of roots colonized by arbuscular mycorrhizal fungi. Mol Plant Microbe Interact 2003; 16:903 - 915
- Ravnskov S, Wu Y, Graham JH. Arbuscular mycorrhizal fungi differentially affect expression of genes coding for sucrose synthases in maize roots. New Phytol 2003; 157:539 - 545
- Schubert A, Allara P, Morte A. Cleavage of sucrose in roots of soybean (Glycine max) colonized by an arbuscular mycorrhizal fungus. New Phytol 2003; 161:495 - 501
- Schaarschmidt S, Roitsch T, Hause B. Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J Exp Bot 2006; 57:4015 - 4023
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu Rev Plant Biol 2006; 57:675 - 709
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK. Extracellular invertase: Key metabolic enzyme and PR protein. J Exp Bot 2003; 54:513 - 524
- Godt DE, Roitsch T. Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol 1997; 115:273 - 282
- Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, Roitsch T, Mueller MJ. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J 2003; 34:363 - 375
- Goetz M, Godt DE, Roitsch T. Tissue-specific induction of the mRNA for an extracellular invertase isoenzyme of tomato by brassinosteroids suggests a role for steroid hormones in assimilate partitioning. Plant J 2000; 22:515 - 522
- Schaarschmidt S, González MC, Roitsch T, Strack D, Sonnewald U, Hause B. Regulation of arbuscular mycorrhiza by carbon: The symbiotic interaction cannot be improved by increased carbon availability accomplished by root-specifically enhanced invertase activity. Plant Physiol 2007; 143:1827 - 1840
- Hause B, Fester T. Molecular and cell biology of arbuscular mycorrhizal symbiosis. Planta 2005; 221:184 - 196
- Javot H, Pumplin N, Harrison MJ. Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles. Plant Cell Environ 2007; 30:310 - 322
- Schaarschmidt S, Kopka J, Ludwig-Müller J, Hause B. Regulation of arbuscular mycorrhiza by apoplastic invertases: Increased invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J 2007; 51:390 - 405
- Herbers K, Meuwly P, Frommer W, Métraux JP, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: Possible hexose sensing in the secretory pathway. Plant Cell 1996; 8:793 - 803
- Baumert A, Mock HP, Schmidt J, Herbers K, Sonnewald U, Strack D. Patterns of phenylpropanoids in noninoculated and potato virus Y-inoculated leaves of transgenic tobacco plants expressing yeast-derived invertase. Phytochemistry 2001; 56:535 - 541
- Cowley T, Walters DR. Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f. sp. hordei. Plant Cell Environ 2002; 25:461 - 468
- Matsuda F, Morino K, Ano R, Kuzawa M, Wakasa K, Miyagawa H. Metabolic flux analysis of the phenylpropanoid pathway in elicitor-treated potato tuber tissue. Plant Cell Physiol 2005; 46:454 - 466
- Herrera-Medina MJ, Steinkellner S, Vierheilig H, Ocampo Bote JA, Garcia Garrido JM. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol 2007; 175:554 - 564