Abstract
Glucose functions in plants both as a metabolic resource as well as a hormone that regulates expression of many genes. Arabidopsis hexokinase1 (HXK1) is the best understood plant glucose sensor/transducer, yet we are only now appreciating the cellular complexity of its signaling functions. We have recently shown that one of the earliest detectable responses to plant glucose treatments are extensive alterations of cellular F-actin. Interestingly, AtHXK1 is predominantly located on mitochondria, yet also can interact with actin. A normal functioning actin cytoskeleton is required for HXK1 to act as an effector in glucose signaling assays. We have suggested that HXK1 might alter F-actin dynamics and thereby influence the formation and/or stabilization of cytoskeleton-bound polysomes. In this Addendum, we have extended our initial observations on the subcellular targeting of HXK1 and its interaction with F-actin. We then further consider the cellular context in which HXK1 might regulate gene expression.
Introduction
HXK-dependent regulation of plant gene expression can involve transcriptional control [maize photosynthetic gene repression],Citation1 translational control [rice α-amylase repression],Citation2 as well as targeted protein degradation [Arabidopsis EIN3 transcription factor turnover].Citation3 How HXK can mediate such a diversity of regulatory processes is largely not understood. Studies with sugar analogs, as well as transgenic and mutant Arabidopsis indicate that the catalytic and regulatory functions of AtHXK1 can be uncoupled from each other.Citation4–Citation6 A current model of plant HXK signaling is that the sensor protein can translocate to the nucleus and somehow modulate transcriptional activity of target gene promoters.Citation7,Citation8
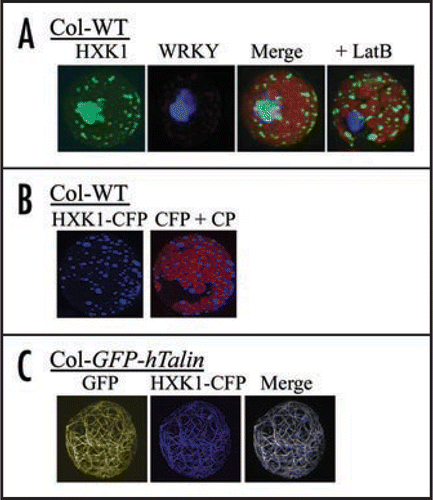
We have shown that AtHXK1 is associated apparently only with mitochondria following transient expression of HXK1-GFP in Arabidopsis or maize leaf protoplasts, after stable expression of HXK1-GFP in Arabidopsis transgenic plants, in isolated pea and Arabidopsis leaf organelles, and by immunostaining of cryofixed leaf tissue of Arabidopsis seedlings.Citation9 Despite numerous efforts, we were not able to observe HXK1 entering or otherwise present in the nucleus. Our inability to detect AtHXK1 in nuclei might have resulted from its being there at very low expression levels. Therefore, we now show data following a prolonged transient expression of HXK1-GFP in mesophyll protoplasts. In this experiment, much of the overexpressed HXK1-GFP aggregated as a large clump within the protoplast (). Such expression might suggest a possible nuclear location. However, co-transfection of a nuclear marker protein (WRKY-CFP) showed that even the aggregated HXK1-GFP did not occur in the nucleus. Staining of Arabidopsis leaf protoplasts using MitoTracker dyes always gives an image with an exact overlap to the fluorescence from transiently expressed HXK1-GFP (observations, reviewed in ref. Citation9). Therefore, the large aggregation of HXK1-GFP fluorescence after prolonged expression is correctly viewed as an induced cluster of mitochondria. Interestingly though, the induced aggregation of mitochondria by HXK1 overexpression was blocked by treatment with LatrunculinB (LatB). LatB disrupts F-actin by binding 1:1 with monomeric G-actin,Citation10 thereby reducing the ability of F-actin to assemble during its normal treadmilling activity. Since plant mitochondria are known to traffic on F-actin,Citation11 this non-aggregating response was not entirely unexpected. However, this experiment does show in a novel way that perturbations of HXK or F-actin can each influence the cellular behavior of the other.
We also have further examined the interaction between HXK1 and F-actin by using a transgenic line that expresses, at a moderate level, the actin binding domains of the hTalin protein fused to an N-terminal GFP tag.Citation12 The use of transgenic reporter lines that express cytoskeleton-GFP marker proteins have proven useful for live cell studies. However, some caution is needed with their use since these typically have F-actin bundling activity and can alter cytoskeletal dynamics when highly expressed.Citation13,Citation14 Nonetheless, these lines have been used to examine responses of the actin cytoskeleton to treatments with stimuli such as ABA,Citation15,Citation16 auxin,Citation17 light,Citation17 glucoseCitation9 and and fungal pathogens.Citation12 We have probed the interaction between HXK1 and F-actin by expressing HXK1-CFP in leaf protoplasts from wildtype (WT) plants or from plants that express GFP-hTalin ( and C). Transfection of WT protoplasts showed that CFP fluorescence occurred in discrete punctate foci, corresponding to an exclusively mitochondrial location. Unexpectedly, transfection of protoplasts from the transgenic plants showed that the HXK1-CFP fluorescence was expressed not in punctate foci, but was associated with most of the actin cables. F-actin was present in this form in the freshly isolated leaf protoplasts and fine mesh filaments were not observed. As one control, the targeting of a cytosol expressed, chloramphenicol-GFP fusion protein was normal when transfected into protoplasts of the transgenic line (personal observations). Also, these protoplasts had normal repression of target promoter activities when tested for glucose signaling by transient expression assay (data not shown; reviewed in ref. Citation9 for assay description).
The hTalin construct used to make the transgenic line in the latter experiment contains 4 modular I/LEWQ actin binding domains.Citation12,Citation18 Interestingly, since tensin proteins in Arabidopsis have this motif, they might also interact with HXK1. Tensins are considered to function as F-actin capping proteins.Citation19 The observed targeting of AtHXK1 to F-actin though might use additional binding surfaces from actin itself, thus requiring an interaction between HXK, the actin binding domains of hTalin, and bound actin. Notably, both actin and HXK have a common evolutionary origin and rather similar tertiary structures that include not only the nucleotide binding site but also the suggested actin binding surface.Citation20,Citation21 There are reports in mammals of HXK interacting either with actin binding proteins or with F-actin.Citation22–Citation24
Recent data from the Sheen lab used yeast 2 hybrid assays, genetic studies and chromatin immunoprecipitation analyses to demonstrate that AtHXK1 can bind indirectly to the 5′–272 bp region of the CAB2 promoter.7 Yet to be established is how HXK enters the nucleus and in which cell types this occurs. Inspection of the primary amino acid sequence of HXK1 does not indicate the presence of a known nuclear localization sequence. However, one of the best conserved motifs outside of the known catalytic domains in yeast, human and Arabidopsis HXKs is a hydrophobic sequence, 325EILRRVLLKMA335 (with reference to AtHXK1, reviewed in ref. Citation26 for alignment). The homologous sequence has been shown to function in hepatic glucokinase as a nuclear export sequence.Citation25 In the case of AtHXK1, one might speculate that if this hydrophobic sequence is functional as an NES, this implies both that HXK1 can somehow enter the nucleus and that it generally might not occur there. Both of these possibilities are consistent with the available data. While hepatic glucokinase can enter the nucleus by interacting with the glucokinase regulatory protein,Citation25 a sequence data base search indicates that Arabidopsis does not have a homolog to the described regulatory protein. Whether HXK-dependent transcriptional repression occurs commonly in mesophyll cells though also is not clear. The apparent difficulty in detecting nuclear localized HXK1-GFP might be due, in part, to this occurring in a restricted number of specific cell types. Notably, Arabidopsis has about 40 different cell types.Citation26 Perhaps transcriptional regulation by AtHXK1 is most important in cell types less abundant than mesophyll cells.
We hypothesize from available data that HXK1 might promote the bundling of actin filaments, while modulated F-actin dynamics might affect the formation and/or stabilization of associated cellular polysomes. In plant cells, a substantial portion of protein synthesis does occur on polysomes bound to F-actin (reviewed in ref. Citation27). Work from Weidner's labCitation28,Citation29 has shown that the level of cytoskeleton bound polysomes in germinating triticale seeds can increase by several-fold in an absolute sense in response to water stress or in a relative sense in response to ABA treatment. They have further demonstrated that the cytoskeleton bound polysomes are the most stable among polysome populations and have suggested that plant hormones might generally modulate the expression of key mRNA binding proteins.Citation30 Furthermore, sugars have been shown to increase the mRNA stability of several growth and stress-related genes,Citation2 as well as to modulate selective mRNA translation (reviewed in ref. Citation8 for discussion). Regulatory sequences in either 3′ or 5′UTRs have been identified in certain cases. Elucidating the roles of glucose, HXK1 and F-actin dynamics is needed to improve our understanding of sugar dependent control of plant gene expression.
Abbreviations:
| CFP | = | cyan fluorescent protein |
| CP | = | chloroplasts |
| GFP | = | green fluorescent protein |
| HXK1 | = | hexokinase1 |
| LatB | = | latrunculinB |
| WT | = | wildtype |
Figures and Tables
Figure 1 Fluorescence images after transfection of different cDNAs into mesophyll protoplasts. Images were collected using a Zeiss LSM 510 confocal laser scanning microscope and band pass filters for GFP and CFP, and a long-pass filter for chlorophyll. (A) Influence of transient overexpression and actin disruption on sub-cellular targeting of AtHXK1-GFP in Arabidopsis protoplasts. Fluorescence of HXK1-GFP or co-transfected WRKY-CFP was visualized after 12 h expression in protoplasts, without or with 2 µM Latrunculin B. The left 3 images are of the same protoplast not treated with LatB, while the image on the far right is of a different protoplast that was treated with LatB. Note that the light-blue image is due to aggregated GFP and does not specifically overlap with the image from CFP fluroscence. (B) Subcellular targeting of transfected AtHXK1-CFP in leaf protoplasts from Col WT. Shown are fluorescence images of transfected HXK1-CFP in Col WT protoplasts, without (left) or with (right) chlorophyll autofluorescence. Note the punctate distribution of blue fluorescence. This pattern is typical for the mitochondrial targeted HXK1.Citation9 CP = chloroplasts. (C) Subcellular targeting of transfected AtHXK1-CFP in leaf protoplasts from Col GFP-hTalin. Shown from left to right are images of wavelength specific fluorescence for GFP-hTalin bound to F-actin, for transfected HXK1-CFP, and for the merged image. Note that the transfected HXK1-CFP now localizes predominantly to F-actin. In the absence of transfected HXK1-CFP, no corresponding blue wavelength fluorescence was observed in the leaf protoplasts.
Acknowledgements
Technical contribution no. 5388 of the Clemson University Experiment Station.
Addendum to:
References
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell 1990; 2:1027 - 1038
- Ho SL, Chao YC, Tong WF, Yu SM. Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol 2001; 125:877 - 890
- Yanasigawa S, Yoo SD, Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signaling in plants. Nature 2003; 425:521 - 525
- Jang JC, Sheen J. Sugar sensing in higher plants. Plant Cell 1994; 6:1665 - 1679
- Jang JC, León P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell 1997; 9:5 - 19
- Moore B, Zhou L, Rolland F, Hal Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 2003; 300:332 - 336
- Cho YH, Yoo SD, Sheen J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 2006; 127:579 - 689
- Rolland F, Baena Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann Rev Plant Biol 2006; 57:675 - 709
- Balasubramanian R, Karve A, Kandasamy M, Meagher RB, Moore Bd. A role for F-actin in hexokinase-mediated glucose signaling. Plant Physiol 2007; 145:1423 - 1434
- Fürstner A, Kirk D, Fenster MDB, Aïssa C, De Souza D, Müller O. Diverted total synthesis: preparation of a focused library of latrunculin analogues and evaluation of their actinbinding properties. Proc Natl Acad Sci USA 2005; 102:8103 - 8108
- Van Gestel K, Köhler RH, Verbelen JP. Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J Exp Bot 2002; 53:659 - 667
- Takemoto D, Jones DA, Hardham AR. GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 2003; 33:775 - 792
- Ketelaar T, Anthony RG, Hussey PJ. Green fluorescent protein-mTalin causes defects in actin organization and cell expansion in Arabidopsis and inhibits actin depolymerizing factor's actin depolymerizing activity in vitro. Plant Physiol 2004; 136:3990 - 3998
- Wilsen KL, Lovy Wheeler A, Voigt B, Menzel D, Kunkel JG, Hepler PK. Imaging the actin cytoskeleton in growing pollen tubes. Sex Plant Reprod 2006; 19:51 - 62
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev 2001; 15:1808 - 1816
- Hwang JU, Lee Y. Abscisic acid-induced actin reorganization in guard cells of dayflower is mediated by cytosolic calcium levels and by protein kinase and protein phosphatase activities. Plant Physiol 2001; 125:2120 - 2188
- Holweg C, Sublin C, Nick P. Arabidopsis myosin XI mutant is defective in organelle movement and polar auxin transport. Plant Cell Physiol 2004; 45:855 - 863
- McCann RO, Craig SW. The I/LWEQ module: a conserved sequence that signifies F-actin binding in functionally diverse proteins from yeast to mammals. Proc Natl Acad Sci USA 1997; 94:5679 - 5684
- Meagher RB, Fechheimer M. Somerville CR, Meyerowitz EM. The Arabidopsis cytoskeltal genome. The Arabidopsis Book 2003; Rockville, MD American Society of Plant Biologists 1 - 26
- van den Ent F, Amos LA, Löwe J. Prokaryotic origin of the actin cytoskeleton. Nature 2001; 413:39 - 44
- Dominguez R. Actin-binding proteins—a unifying hypothesis. Trends Biochem Sci 2004; 29:572 - 578
- Murata T, Katagiri H, Ishihara H, Shibasaki Y, Asano T, Toyoda Y, Pekiner B, Pekiner C, Miwa I, Oka Y. Co-localization of glucokinase with actin filaments. FEBS Lett 1997; 406:109 - 113
- Wagner O, Zinke J, Dancker P, Grill W, Bereiter Hahn J. Viscoelastic properties of F-actin, microtubules, F-actin/α-actinin, and F-actin/hexokinase determined in microliter volumes with a novel nondestructive method. Biophys J 1999; 76:2784 - 2796
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 2003; 424:952 - 956
- Shiota C, Coffey J, Grimsby J, Grippo JF, Magnuson MA. Nuclear import of hepatic glucokinase depends upon glucokinase regulatory protein, whereas export is due to a nuclear export signal sequence in glucokinase. J Biol Chem 1999; 274:37125 - 37130
- Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, Vorwerk S, Youngs H. Toward a systems approach to understanding plant cell walls. Science 2004; 306:2206 - 2211
- Azama K, Abe S, Sugimoto H, Davies E. Lysine-containing proteins in maize endosperm: a major contribution from cytoskeleton-associated carbohydrate-metabolizing enzymes. Planta 2003; 217:628 - 638
- Kosowska M, Fraczek E, Amarowicz R, Karamac M, Abe S, Weidner S. Water-deficitinduced changes in cytoskeleton-bound and other polysomal populations in embryonic tissue during tritical caryopsis germination. Acta Physiol Plant 2004; 26:67 - 74
- Weidner S, Łukaszewicz D, Amarowicz R. Significant role for polysomes associated with the cytoskeleton in the control of protein synthesis during germination of tritical caryopses in the presence of abscisic acid. Acta Physiol Plant 2000; 22:185 - 193
- Weidner S, Kazarnowicz M, Fraczek E, Amarowicz R, Karamac M. Exogenous abscisic acid increases stability of polysomes in embryos of tritical caryopses during germination. Acta Physiol Plant 2006; 28:627 - 634