Abstract
By endocytosis eukaryotic cells can take up extracellular components and/or plasma membrane proteins for further delivering to endosomes. Although in animal cells different endocytic pathways were identified based on the requirement of a clathrin coating for vesicle internalization, endocytosis in plant cells still require to be fully characterized. The use of positively and negatively charged nanogold in combination with Ika, an inhibitor of the clathrin-dependent endocytosis, allowed to dissect the endocytic pathway and revealed the presence of clathrin-dependent and clathrin-independent degradative pathways.
Introduction
During differentiation, cells acquire different morphologies, typically by delivering new plasma membrane (PM) and cell wall material through the exocytotic pathway.Citation1 Angiosperm pollen tubes represent attractive models to investigate mechanisms involved in asymmetrical patterns of growth and cell membrane recycling. Pollen tubes follow a precise, regulated tip growth pattern based on the transport and accumulation of post-Golgi secretory vesicles (SVs) at the extreme tip where, upon the fusion with a restricted region of the PM, they secrete cell wall material and provide new segments of PM. However, SV flux causes a membrane contribution much higher than that required for pollen tube elongation. Pollen tube growth is therefore the result of balanced exocytotic and endocytotic processes, where the endocytosis is involved in regulating the protein/lipid composition of the PM,Citation1–Citation4 the excess PM turnover and the internalization of external molecules which, “in vivo”, play a role in regulating pollen tube growth.5 In animals, beside the well characterized clathrin-dependent endocytosis, internalization mechanisms that do not require the formation of a clathrin coat have been identified and shown to coexist in the same cell.Citation6 A sequence coding for a clathrin heavy chain-like polypeptide was identified by screening a soybean cDNA library. Both immunofluorescence studies by using a monoclonal antibody to plant CHCCitation7 and the observation of coated vesicles in regions proximal to the tipCitation8 suggested that a mechanism of clathrin-dependent endocytosis act in pollen tubes. However, the presence of clathrin-independent internalization pathways were not described in plants.
The aim of our approach to the endocytic process was to discover if more internalization pathways could be present in pollen tube and to describe the morphology of clathrin-dependent and -independent endocytosis by electron microscopy.
Internalization Pathways Occurring in the Subapical and in the Apical Plasma Membrane are Responsible for Recycling and Degradation
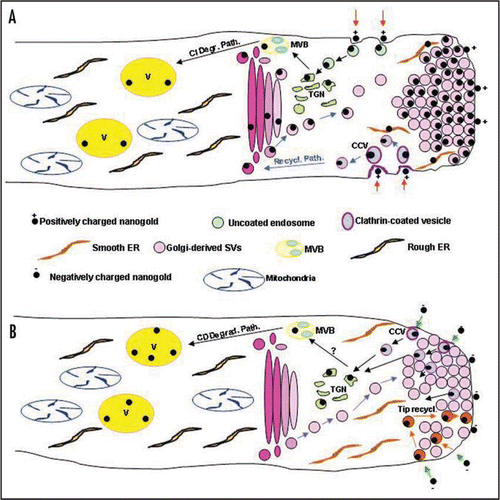
In the last few years, Parton and colleagues (2001)Citation9 analysed the endocytic process in pollen tubes of several species by using FM4-64.Citation10 This approach showed that internalized PM was accumulated at the tip where a typical V-shaped fluorescent region was observed, suggesting that most of the internalized PM was redirected into the secretory pathway. This was in agreement with the hypothesis that in pollen tube endocytosis could have the main function to recycle the excess of PM secreted during tube growth.Citation11 However, a detailed analysis of the process by using charged nanogold showed a more complex scenario (see models in ). Positively and negatively charged nanogold are internalized in different areas and have different destinies: positively charged nanogold uptake occurs in the flanks of the tube, at the level of the organelle rich zone, while negatively charged nanogold is up taken in the clear zone. This aspect was confirmed by quantisation analysis of FM4-64 entrance in the tip (5 µm) and in the subapical region (up to 30 µm from the tip): endocytosis in the flanks appeared to be double respect to the tip. This observation allow to reconsider the common idea that vesicles accumulated in the clear zone are Golgi-derived SVs, instead we have to realize that both secretory and endocytic vesicles contribute to the typical V-shaped fluorescent accumulation at the tip.
Interestingly both probes revealed a degradation pathway involving elements of the trans Golgi network (TGN) that, in plants, seems to the first sorting station for internalized material ( and B).Citation12 Then positively charged nanogold is observed within multivesicular bodies-like organelle (MVBs) (, black arrows) while negatively charged nangold was not seen inside compartments similar to MVBs ( B, black arrows). This observation suggested that two degradation pathways, involving different compartments could act in pollen tubes. Interestingly, transport of positively charged nanogold involved all compartments of Golgi bodies before to be recycled in secretory vesicles (). On the other hand, negatively charged nanogold revealed the presence of a recycling process limited to the clear zone that do not involve the Golgi apparatus (, orange arrows). The latter observation fit well with data showing that enzymes involved in regulating the differential lipid composition in apical PM are repositioned by endocytosis in the clear zone as the tube elongate.Citation4
Clathrin-Dependent and -Independent Degradation Pathways
Recently Dhonukshe et al. (2007)Citation13 reported data on the constitutive clathrin dependent endocytosis of PIN efflux carriers in Arabidopsis protoplasts and suggested that clathrin-dependent internalization could be the predominant system in plants. However the occurrence of clathrin-independent mechanisms, in specialized cells as the pollen tubes could not be excluded. Our data on the presence of clathrin-independent pathways confirm data reported in the study of Derksen et al. 1995Citation8 in which an accurate calculation of the retrieved membrane was done. This led to the conclusion that SVs deliver 430 µm2/min of which only 50 µm2 is used for expansion, leaving an excess of 380 µm2. The endocytic activity behind the tip, taken at its maximum of 225 µm2/min, cannot retrieve a membrane surface of this size. Since in this case only the area delimited by coated pits or coated vesicles was considered, part of internalization could actually occur by clathrin-independent mechanisms. Our data using IkaCitation14,Citation15 suggested that internalisation of PM to be recycled to exocytosis was clathrin-dependent (, purple vesicles) as well as the degradation pathway shown by the negatively charged nanogold (, purple vesicles). On the other hand, the degradation pathway carrying positively charged nanogold was clathrin-independent, so confirming observations reported above: in pollen tubes two different internalisation mechanisms lead to distinct degradative pathways, that could have different functions in the physiology of the tube. We have to consider that pollen tube growth, in vivo, is tightly regulated by its interaction with style molecules. The study of endocytosis in pollen tubes open new perspective to investigate on pollen tube-style interaction in vivo. Particularly in the self-incompatibity process which characterize N. alata, where the compatibility/incompatibility of pollen tubes growing in the style seems to be determined by the sequestration in vacuole/like compartments of S-RNases which are internalized in both kind of tubes.Citation5 However, in the case of incompatible pollen, S-RNases escape from vacuole-like vesicles and to function as degradation enzymes, on the other hand S-RNases remain sequestred within vacuoles and pollen tubes can growth through the style after a compatible pollination.Citation5 If the internalisation of S-RNases follow different mechanisms in compatible and incompatible pollinations is not known at the present, however, it seems that the mechanism of internalisation (clathrin-dependent or -independent) could predict the destiny of the internalised materials into different transport pathways and it would be of great interest to study the mechanism by which S-RNases can escape their vacuole-like compartment in order to become toxic for the tube after the incompatible pollination.
Figures and Tables
Figure 1 Models showing endocytic pathways revealed by positively (A) and negatively (B) charged nanogold. (A) Positively charged nanogold seems to be internalised in the organelle rich zone both by clathrin-dependent (purple vesicles) and -independent endocytosis (green vesicles). In the first case endocytic vesicle are recycled to exocytosis through the Golgi apparatus (blue arrows), in the other case vesicles are transported to the TGN and then directed to the degradative pathway (black arrows). After 2 hours incubation gold particles are seen also within smooth ER between SVs in the clear zone. (B) Internalization of negatively charged nanogold is internalised in the clear zone. A clathrin-dependent internalisation seems to be responsible for the endocytic pathway leading to vacuoles (purple vesicles, black arrows). It is not know at the moment if internalisation of vesicles cycling in the tip region (orange vesicles) requires the formation of the clathrin coat.
Addendum to:
References
- Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 2001; 17:159 - 187
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH. Rac homologues and compartmentalized phosphatidylinositol 4, 5-biphosphate act in a common pathway to regulate pollen tube growth. J Cell Biol 1999; 19:317 - 330
- Potocky M, Elias M, Profotova B, Novotna Z, Valentova O, Zarsky V. Phosphatidic acid produced by phospholipase D is required for tabacco pollen tube growth. Planta 2003; 217:122 - 130
- Helling D, Possart A, Cottier S, Klahre U, Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 2006; 18:3519 - 3534
- McClure BA, Franklin-Tong V. Gametophytic self-incompatibility: Understanding the cellular mechanisms involved in “self ” pollen tube inhibition. Planta 2006; 224:233 - 245
- Kirkam M, Parton RG. Clathrin-independent endocytosis: New insights into caveolae and non caveolar lipid rafts. Bioch Biophys Acta 2005; 1745:273 - 286
- Blackbourn HD, Jackson PA. Plant clathrin heavy chain: Sequence analysis and restricted localisation in growing pollen tubes. J Cell Sci 1996; 109:777 - 789
- Derksen J, Rutten T, Lichtscheidl IK, Dewin AHN, Pierson ES, Rongen G. Quantitative analysis of the distribution of organelles in tobacco pollen tubes: Implication for exocytosis and endocytosis. Protoplasma 1995; 188:267 - 276
- Parton RM, Fisher-Parton S, Watahiki MK, Trewavas AJ. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci 2001; 114:2685 - 2695
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Juenemaitre B. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 2004; 214:159 - 173
- Steer MW, Steer JL. Pollen tube tip growth. New Phytol 1989; 111:323 - 358
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+ ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006; 18:715 - 730
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carrier in Arabidopsis. Curr Biol 2007; 17:520 - 527
- Hasumi K, Shinohara C, Naganuma S, Endo A. Inhibition of the uptake of oxidazed low-density lipoprotein in macrophage J774 by the antibiotic ikarugamycin. Eur J Biochem 1992; 205:841 - 846
- Luo T, Fredericksen BL, Hasumi K, Endo A, Garcia V. Human immunodeficiency virus type 1 Nef-induced CD4 cell surface downregulation is inhibited by ikarugamycin. J Virol 2001; 75:2488 - 2492