Abstract
Targeted delivery of immotile sperm through growing pollen tubes is a crucial step in achieving sexual reproduction in angiosperms. Unlike diffuse-growing cells, the growth of a pollen tube is restricted to the very apical region where targeted exocytosis and regulated endocytosis occur. The plant-specific Rho GTPases, Rops, are central organizers for pollen tube polarity. Through effector binding, Rops regulate the tip-focused Ca2+ gradient and the actin cytoskeleton during pollen tube growth. Therefore, understanding the spatiotemporal regulation of Rop activity would reveal how establishment and maintenance of tube polarity as well as re-orientation of the growth axis are accomplished. Recent findings indicated that two feedback loops may be fundamental in maintaining a fine-tuned and dynamic Rop activity. The concerted activities of RopGAP and RopGDI prevent lateral diffusion of activated Rop, restricting Rop activity to the apical plasma membrane. Conversely, pollen receptor kinases (PRKs) and RopGEFs positively feedback regulate Rop activity through protein binding and membrane recruitment. Feedback loops would also be essential for pollen tube re-orientation. Shallow extracellular cues amplified by concerted activities of feedback loops would lead to asymmetric activation of Rop and result in tube re-orientation.
Plant-Specific Rho GTPases, Rops/Racs, Are Central Organizers During Pollen Tube Growth
Polarized growth of pollen tubes is a critical step for sexual reproduction of angiosperms. Pollen tubes are initiated upon landing on the stigmatic surface. Growing tubes penetrate deep inside the pistil to deliver immotile sperm to the embryo sac, a process that requires the maintenance of polarity over a certain distance and the perception of guidance cues.Citation1 How such a targeted delivery can be accomplished is a fascinating area not only because of its agricultural significance, but because its understanding will shed more light on the evolutionary origin of signaling cascades that control cell polarization and environmental sensing.
As in other polarized cells, such as neurite growth cones and neutrophils, plant-specific Rho GTPases (designated Rops/Racs) are central organizers of intracellular activities. Rho GTPases act as molecular switches, alternating between a GTP-bound active and a GDP-bound inactive state.Citation2 In addition, membrane association of Rho GTPases is essential for their functionality. Rop associates with the apical plasma membrane in pollen tubes,Citation3 where targeted exocytosis and regulated endocytosis occur to drive rapid growth.Citation4 The GTP-bound Rop regulates both a tip-focused Ca2+ gradient and apical actin cytoskeleton organization through two antagonistic effector pathways.Citation5 Constitutively active Rop resulted in isotropic growth,Citation6,Citation7 i.e., radial expansion rather than tip growth, whereas reduced Rop activity inhibited tube growth.Citation6–Citation8
Evolutionarily Conserved Negative Feedback Loop
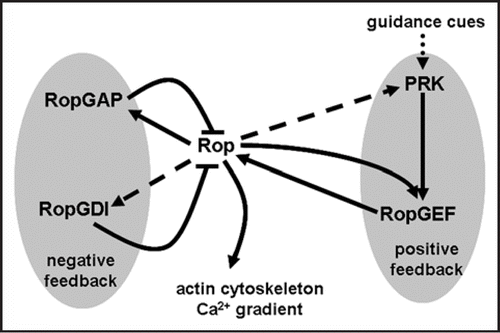
Considering the critical roles that Rops play, the key to pollen tube growth would be a fine-tuned and dynamically regulated Rop activity. Recent advances indicated the existence of both negative and positive feedback loops in spatiotemporally regulating Rop activity. The negative feedback loop includes the GTPase activating proteins (GAPs) and the guanine nucleotide dissociation inhibitors (GDIs). RopGAPs accelerate the intrinsic GTPase activity of Rop, thereby preventing the lateral diffusion of activated Rop during tube growth.Citation9,Citation10 The localization and activity of RopGAPs are in turn regulated by Rop through a CRIB domain (Cdc42/Rac interactive binding motif) within RopGAPs, which facilitates Rop-binding and promotes GAP activity.Citation9,Citation10 RopGDIs sequester GDP-bound Rop in the cytosol where GTP is abundant.Citation2 Because RopGDIs preferentially bind to prenylated, GDP-bound Rop at the plasma membrane,Citation11 they are critical to actively remove Rop at the junction between the apical and subapical plasma membrane during pollen tube growth, and by doing so, restrict the membrane distribution of Rop to the apical region. Both the catalytic domains of RopGAPs and RopGDIs are evolutionarily conserved and a similar negative feedback loop also exists in metazoans.Citation12
Plant-specific Positive Feedback Loop
In contrast to the evolutionarily conserved negative feedback loop, plants evolved a distinct positive feedback loop for controlling Rop activity. Polarized metazoan cells generally use transmembrane G protein-coupled receptors (GPCRs) to sense extracellular stimuli and Dbl-type RhoGEFs to relay signals for spatiotemporal Rho activation.Citation12 No conventional GPCRs have been identified in plants. Instead, receptor-like kinases are ideal candidates for transmembrane signaling relays in plants, due to their large numbers and diversified extracellular domains.Citation13 Plants also do not have homologs of Dbl-type RhoGEFs. Rather, a plant-specific novel class of RopGEFs has recently been characterized.Citation14–Citation16 RopGEFs contain a central catalytic domain (PRONE) flanked by regulatory domains.Citation14,Citation15,Citation17 We recently showed that pollen-specific RopGEFs interact with pollen receptor kinases (PRKs).Citation16,Citation17 Such an interaction releases the C-terminal inhibition of RopGEF activity.Citation17 The interaction between PRKs and RopGEFs recruits RopGEFs to the plasma membrane, thus serving as a positive feedback loop to maintain continued activation of Rop at the apical plasma membrane. There is as yet no evidence showing direct regulation of PRKs by Rop. However, given that PRKs are transmembrane receptors, it is plausible that the spatiotemporal membrane distribution and concentration of PRKs are influenced by vesicle trafficking, which in turn is regulated through Rop-mediated Ca2+ gradient maintenance and actin polymerization.Citation5
It is not surprising that components in the negative feedback loop are conserved during evolution while those in the positive feedback loop are not. The negative feedback loop is only involved in self-organizing cycles for cell polarization, while the positive feedback loop is also responsible for receiving and responding to ever-changing extracellular cues. It has always been an intriguing thought that pollen tubes can sense and respond to a myriad of signaling cues. How such a complexity can be achieved may also lie in the positive feedback loop. Multiple PRKs,Citation18 RopGEFsCitation17 and RopsCitation19 are expressed in pollen. Supposing there are interaction specificities conferred by extracellular stimuli, many different combinations of these three components can be envisioned.
Pollen Tube Growth: It is All About Coordinated Activities of Feedback Loops
We propose that coordinated activities of feedback loops are fundamental to the establishment, maintenance and re-localization of spatially restricted Rop activity during pollen tube growth. In this model (), random membrane association of Rop before pollen germination results in small concentration differences along the pollen plasma membrane. Feedback loops amplify such slight differences to establish a stable growth axis in which Rop resides at the apical plasma membrane. During rapid growth, when there is no need for re-orientation, the growth axis is constant, as is the case during in vitro pollen tube growth.Citation20 Such a self-organizing property of pollen tubes requires dynamically restricted apical Rop localization, made possible by feedback loops. In addition, a positive feedback loop composed of PRKs and RopGEFs has the ability to perceive shallow gradients of extracellular stimuli. To transduce the perceived signals, the positive feedback loop asymmetrically activates Rop, which then is stabilized by the negative feedback loop. A spatiotemporal switch of Rop activity at plasma membrane finally redirects intracellular activities, leading to pollen tube re-orientation.
Abbreviations
| RopGEF | = | guanine nucleotide exchange factor for Rop |
| RopGAP | = | GTPase activating protein for Rop |
| RopGDI | = | guanine nucleotide dissociation inhibitor for Rop |
| PRK | = | pollen receptor kinase |
| CRIB | = | Cdc42/Rac interactive binding motif |
| GPCRs | = | G protein-coupled receptors |
| RLKs | = | receptor like kinases |
Figures and Tables
Figure 1 Feedback loops of Rop control polarized growth of pollen tubes. As described in the text, RopGAPs inhibit Rop activity by accelerating intrinsic GTPase activity while RopGDIs extract prenylated, GDP-bound Rop from the membrane for its recycling to the apical plasma membrane. Both are regulated by Rop through direct interactions, forming a negative feedback loop. PRKs interact with RopGEFs to facilitate their membrane association. The apical plasma membrane-localized Rop and membrane PRKs bind to different domains of RopGEFs, forming a positive feedback loop that regulates Rop activity. PRKs may be regulated by Rop-mediated vesicle trafficking, shown with a dashed arrow. Extracellular cues directly alter the activity of the positive feedback loop, resulting in tube re-orientation.
Addendum to:
References
- Johnson MA, Preuss D. Plotting a course: multiple signals guide pollen tubes to their targets. Dev Cell 2002; 2:273 - 281
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 2002; 16:1587 - 1609
- Lin Y, Wang Y, Zhu JK, Yang Z. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 1996; 8:293 - 303
- Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 2001; 17:159 - 187
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 2005; 169:127 - 138
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua NH. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol 1999; 145:317 - 330
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 1999; 11:1731 - 1742
- Lin Y, Yang Z. Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell 1997; 9:1647 - 1659
- Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 2006; 18:3033 - 3046
- Wu G, Li H, Yang Z. Arabidopsis RopGAPs are a novel family of rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for rop-specific GTPase stimulation. Plant Physiol 2000; 124:1625 - 1636
- Klahre U, Becker C, Schmitt AC, Kost B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant J 2006; 46:1018 - 1031
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 2004; 116:167 - 179
- Shiu SH, Bleecker AB. Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 2001; 2001:22
- Berken A, Thomas C, Wittinghofer A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 2005; 436:1176 - 1180
- Gu Y, Li S, Lord EM, Yang Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell 2006; 18:366 - 381
- Kaothien P, Ok SH, Shuai B, Wengier D, Cotter R, Kelley D, Kiriakopolos S, Muschietti J, McCormick S. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J 2005; 42:492 - 503
- Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci USA 2007; 104:18830 - 18835
- Kim HU, Cotter R, Johnson S, Senda M, Dodds P, Kulikauska R, Tang W, Ezcura I, Herzmark P, McCormick S. New pollen-specific receptor kinases identified in tomato, maize and Arabidopsis: the tomato kinases show overlapping but distinct localization patterns on pollen tubes. Plant Mol Biol 2002; 50:1 - 16
- Cheung AY, Chen CY, Tao LZ, Andreyeva T, Twell D, Wu HM. Regulation of pollen tube growth by Rac-like GTPases. J Exp Bot 2003; 54:73 - 81
- Lord E. Adhesion and cell movement during pollination: cherchez la femme. Trends Plant Sci 2000; 5:368 - 373