Abstract
The main objective of this work is to find out whether aluminum (Al) toxicity and Ca depletion cause cell death of tobacco cells via similar sequence of events. Tobacco cell suspension culture exhibited maximum fresh weight in the presence of a wide range of Ca concentrations between 0.1-1.0 mM whereas higher concentrations (> 1.0-5.0 mM) gradually lowered cell fresh weight. However, this decrease in fresh weight does not imply a negative impact on cell viability since cell growth recommenced in fresh MS medium with rates mostly higher than those of low Ca. In addition, high Ca seems to be crucial for survival of Al-treated cells. On the other side, tobacco cells exhibited extreme sensitivity to complete deprivation of Ca. Without Ca, cells could not survive for 18 h and substantially lost their growth capability. Evans blue uptake proved membrane damage of Ca-depleted same as Al-treated cells; relative to maintained membrane intactness of calcium-supplemented (control) ones. Percentage of membrane damage and the growth capability (survival) of tobacco cells exhibited a clear negative correlation. Alterations in growth (fresh weight per aliquot) could not be ascribed neither to cell number nor to decreased dry matter allocation (dry weight/fresh weight percentage) but was mainly due to decreased cellular water content. In this context, Ca-depleted cells lost about half their original water content while 100 μM Al-treated ones retained most of it (ca 87%). This represented the single difference between the two treatments (discussed in the text). Nevertheless, such high water content of the Al-treated cells seems physiologically useless since it did not result in improved viability. Similarities, however, included negligible levels of growth capability, maximum levels of membrane damage, and comparable amounts of NO3- efflux. As well, both types of treatments led to a sharp decline in osmotic potential that is, in turn, needed for water influx. The above-mentioned sequence of events, induced by Al application looks, to a great extent, similar to Ca depletion syndrome leading finally to cell death of tobacco cells.
Introduction
Aluminum, the third element in the earth's crust, is released from soil complexes into the ionic and thus toxic form that inhibits plant growth under soil acidity. Because of its pH-dependent solubility, aluminum toxicity is most severe in soils with low base saturation, poor in Ca and Mg.Citation1 Aluminum chemistry preferentially leads to its binding to oxygen-containing moieties such as carboxyl, phosphate groups and amines in acidic pH. Therefore, it is generally accepted that the apoplastic region is the primary target of aluminum toxicity (with abundant carboxyl groups on the cell walls). Despite this established localization of Al in the plant cell wall,Citation2 it has been debated whether the mere electrostatic binding of aluminum to the cell walls is unlikely a significant mechanism in aluminum toxicity (reviewed in ref. Citation1). Associated protoplasm is necessary for aluminum to accumulate in a non-exchangeable manner, including aluminum in the cell wall (e.g., the newly synthesized wall material) since Al is not observed to accumulate in the walls of plasmolyzed cells.Citation3 Sites of aluminum toxicity and tolerance mechanisms have been intensively and extensively reviewed in refs. Citation4 and Citation5. Takabatake and ShimmenCitation6 specified the site of aluminum action as the outer surface of the plasma membrane in the first 24 hrs of treatment, inducing changes in its physical and structural properties.Citation7 Taylor et al.,Citation8 reported that in Chara corallina membrane transport of Al occurs within minutes of exposure and is supported by subsequent sequestration in the vacuole. Consequently, aluminum significantly affects the ion flux across the plasma membrane,Citation9 the permeability of the plasma membrane to nonelectrolytesCitation10 as well as decreases the fluidity of membranes.Citation11 Aluminum-tolerant species exudate organic acids to bind aluminum and thus reduce the amount that reaches the membrane. However, two pieces of evidence may prove aluminum influx into the cytoplasm.Citation12 These are aluminum entry through a Ca2+-channel-like pathway (sensitive to Ca2+ channel blockers Verapamil and La3+) and aluminum inhibition of K uptake by blocking its channel at the cytoplasmic side of the plasma membrane. Furthermore, Al-induced oxidative stress has been most commonly attributed to alterations in membrane structure, which then favor radical chain reactions mediated by Fe resulting in the formation of lipid peroxides.Citation13–Citation15
Of particular concern in the scope of this work is aluminum interaction with Ca2+. Earlier, Foy et al.,Citation16 reported that the symptoms of aluminum toxicity are similar to those of Ca2+ deficiency. In this respect, aluminum may affect the uptake of Ca2+ and other ionsCitation12,Citation17 or signal transduction and calcium homeostasis.Citation18 Subsequently, aluminum toxicity has frequently been linked to CalciumCitation18 either because of Al3+-induced perturbations in cellular Ca2+ metabolism or because of Ca2+-mediated amelioration of Al3+ toxicity.Citation19 In addition, aluminum induces alterations in the structure of calmodulin, the chief mediator of intracellular Ca2+ signaling.Citation20 Phosphoinositidemediated signal transduction involving Ca2+ as an intracellular messenger is a primary site of aluminum toxicity.Citation21 Ca2+ acts as a secondary messenger for signals of various stimuli such as light, water stress, salinity and toxic elements (reported e.g., by Sanders et al.)Citation22 Changes in intracellular free calcium levels may be ambiguous in the sense that whether a result or the beginning of response.Citation1
In acidic conditions the ionization and solubility of both Al3+ and Ca2+ are enhanced and their interaction becomes more probable. In this respect, it has been found that the effect of Al3+ on calcium transport is rapid, reversible and kinetically consistent with competitive inhibition.Citation23,Citation24 These authors concluded, then, that Al interferes with calcium homeostasis. Vice versa, increasing Ca2+ concentration in the growth medium may lead to an amelioration of Al-induced metabolic disorders.Citation25 However, Takano and ShimmenCitation26 found that Ca2+ did not show an ameliorative effect against AlCl3 inhibition, but it was effective in mediating recovery after the AlCl3 treatment. They suggested the possibility that Ca2+ easily dissociates aluminum bound to the exterior of the plasma membrane in the absence of AlCl3.
Based on the literature discussed, aluminum and Ca2+ may compete for at least two sites; cell walls and cell membranes. The work presented here was conducted to resolve the hypothesis that Al toxicity and Ca depletion might induce similar sequence of events leading finally to cell death. Thus a comparison between growth and growth capability, water content, membrane integrity, osmotic potential and NO3− efflux in relation to cell viability has been conducted.
Results and Discussion
Toxic impacts of aluminum have been reported to involve almost all cellular components from cell walls to nuclei. MatsumotoCitation4 and Matsumoto et al.,Citation5 reviewed, intensively and extensively, sites and mechanisms of aluminum toxicity. A prominent feature of Al action, regarding this work, is its competition with (or replacement of) Ca on cell walls and membranes. The symptoms of Al toxicity are similar to those of Ca deficiency as it has been reported by Foy et al.Citation16 Therefore, this work has been conducted to compare the sequence of events leading to cell death in Al-treated and Ca depleted tobacco cells. Cultured plant cells have several advantages over higher plants not only because each cell in the suspension is directly subjected to Al thus making experimental conditions more controllable, but also because it is a more flexible system to study the aspects of Al toxicity at the cellular and molecular levels. Furthermore, cell suspension is more physiologically homogeneous than whole plants with barriers such as translocation being avoided.
The growth and maintenance medium of tobacco cells contains 3 mM Ca. As well, the simple calcium-sucrose medium that is used for the application of aluminum treatment contains the same amount of calcium. Since the concentration of 3 mM Ca is much higher than that in the soil, we surveyed the effect of a series of Ca concentrations (0.05–5.0 mM) seeking a concentration-dependent maximum growth of tobacco cells. Second, the same series of Ca concentrations was used in combination with 100 µM Al to elucidate any protective or ameliorative role of Ca against Al toxicity. Such a role has been assessed primarily by cell fresh weight at the end of 18 h treatment and by the determination of growth capability; a measure of surviving cells and their capability to recommence growth (see materials and methods). The results, presented herein, showed that calcium concentrations as low as 0.1 up to 1.0 mM induced more or less similar (maximum) fresh weight of tobacco cells (). Higher levels (2, 3, 4 or 5 mM), however, relatively lowered the fresh weight but seemingly without affecting their viability i.e., growth capability (). When combined with Al, there was no protective effect of any calcium concentration for tobacco cells throughout the growth period (18 h); assessed by no pronounced increase in fresh weight of the starting inoculum. The preferential role of low and high Ca is clearly observed in growth capability of Al-treated cells since high levels of Ca (>1.0 mM) seem to be crucial for the survival (growth capability) while low Ca-treated cells completely lost such capability (). Similar effectiveness of Ca in mediating recovery after Al treatment has been also noticed by Takano and ShimmenCitation26 working with internodal cells of Chara corallina. They suggested, then, that Ca could remove Al bound, during the treatment, to the exterior of the membranes. In addition, the Al-induced inhibition of Ca uptake in wheat cultivar Scout 66 was rapidly reversed after Al was removed from the solution.Citation32 Aluminum toxicity is strongly influenced by H+ and other cations in the rhizosphere. Negative charges on the cell surface of the root accumulate Al3+, and amelioration is affected by treatments that reduce the negativity of the cell surface electrical potential (zeta potential) by charge screening or cation binding.Citation33 In accordance with this idea, alleviation of Al toxicity is achieved by the addition of Ca2+, thus restricting the accessibility of Al3+ to the membrane. In this regards, Ahn et al.,Citation34 reported the depolarization of zeta potential by Al. The depolarization of zeta potential by Ca2+ in vitro was slight (10%) compared with Al3+. This indicates that a much higher concentration of Ca2+ than Al3+ is needed to alleviate Al toxicity.Citation25
Calcium depletion of tobacco cells was found to be as lethal to tobacco cells as aluminum toxicity. Therefore, the coming part of this work was conducted to find out whether a similar sequence of events was leading to cell death by aluminum toxicity and calcium depletion. In this case, aluminum was combined with low calcium concentration (0.5 mM) to be closer to field concentrations with minimum protective effect. It is noteworthy to denote that alterations in cell multiplication and dry matter allocation should be excluded as limiting factors in growth (fresh weight per aliquot). The results presented in table 1 display that alterations in cell number (based on the counts of isolated protoplasts) were minor among Al-treated, Ca-depleted and control cultures. Also, no considerable alteration in dry weight/fresh weight percentage (dry matter allocation) has been observed in Al-treated and control cells (ca. 6.5%). However, in Ca-depleted ones this ratio was higher (ca. 8.5%) due to loss of a considerable proportion of their water content.
In this context, it seems that a major role of calcium is to trigger water influx into tobacco cells. If water content on cell basis in Ca-depleted and control (Ca-supplemented) cells is being compared, the depleted cells preserved about half (54%) the control value (). In this respect, tobacco cells exhibited extreme sensitivity to complete depletion of Ca and may represent a relevant material for complete Ca-depletion studies that are extremely difficult to perform in other plant materials (e.g., higher plants or algae). However, Al-treated cells retained water almost as much as those of control cells (ca. 87%). Despite such a discrepancy in water content, growth capability () of both Al-treated and Ca-depleted cells was completely lost (less than 10% that of the control) indicating cell death in both cultures. Therefore, high water content in Al-treated cells compared with Ca-depleted cells seems to be physiologically meaningless in maintaining cell viability. Membrane damage (discussed later) could not be the proper justification for such a discrepancy in water content since Ca-depletion and aluminum treatment similarly led to membrane damage and loss of growth capability, evoking another explanation. Preservation rather than continued uptake of cellular water in aluminum-treated cells might be ascribed to callose deposition that characterizes Al-treated cells. Callose deposition, in tobacco cells, occurred as a very early response (within 1 h) to Al imposition.Citation35 Al treatment increases cytoplasmic free Ca (due to release from cell walls and membranes and may be with enhanced Ca influx via damaged membranes). High Ca concentration, in turn, represents a signal triggering callose deposition.Citation4 Moreover, β-1, 3 glucan synthase located in the plasma membrane is strictly dependent on Ca in vitro and played a role in the deposition of callose.Citation36 Callose deposition per se can be considered a marker of cell death.Citation37 More important is its action as a sealing system inhibiting water and mineral uptake.Citation38 Aluminum-induced callose inhibited symplasmic and apoplastic transport in wheat and corn roots.Citation39,Citation40 As a sealing system, callose in Al-treated cells not only inhibits water influx but also might preserve cellular water content from efflux to the outer medium while Ca-depleted cells, lacking callose, could not prevent water loss. Efflux of water is probably a result of an abolished osmotic gradient between cell sap and outer medium (discussed later). From the above- discussed results regarding water content, it can be concluded that Ca depletion as well as Al treatment enhanced cell death of tobacco cells despite discrepancy in their water content.
Evans blue uptake proved membrane intactness of calcium- supplemented (control) cells since they retained only 15% of the dye. Ca-depleted and Al-treated cells exhibited similarly considerable higher values (about 3 times of control cells) of Evans blue uptake (). This high level of dye retention indicates that membranes of both cultures were severely deteriorated implying cell death. The results presented in and , clearly show that percentage of membrane damage and loss of growth capability are correlated. Formerly in this lab, it was found that Al did not enhance membrane damage of tobacco cells unless Fe(II) was addedCitation35 as Al sensitizes the membranes to Fe(II)-mediated lipid peroxidation which, in turn, causes loss of membrane integrity. The present results clearly show that 100 µM Al in the presence of low Ca (0.5 mM) leads to loss of membrane integrity and enhanced Evans blue uptake without Fe (II) mediation.
Ca-depletion and 100 µM Al considerably and similarly lowered the osmotic potential of tobacco cells (). A role for aquaporin has been excluded (data not shown); hence the osmotic gradient plays the key role in water uptake. Since the uptake of water depends exclusively on cellular osmotic potential, the Al-tolerant cell line ALT301 has been studied only in this experiment to find out if the characteristic sensitivity of the cell line SL is due to its inability to maintain higher osmotic potential. However, the two cell lines (the sensitive and the tolerant) responded similarly to aluminum with relatively higher values in the tolerant one; indicating that the osmotic potential is not correlated to Al tolerance. As long as the osmotic potential depends, in turn, on cytosolic solutes it can be concluded that Al toxicity surpasses the apoplastic region and membranes into the cytoplasm. As well, Ca depletion triggered the decrease in osmotic potential of the cell sap very early after only 6 h following depletion while control (Ca-supplemented) cells continued to elevate their osmotic potential and hence take up water. In the cell line SL, the control value of osmotic potential was 260 mOsm/Kg, dropped to about 70% this value in Al-treated or Ca-depleted cells. In a detailed study concerning the kinetics of osmotic water permeability, it was confirmed that such a drop in osmotic gradient between cell sap and outer medium played a major role in inhibiting water influx (data not presented). Kinetics of decreased osmotic potential coincided with inhibited fresh weight increment; both started to decrease after 6 h following Al treatment or Ca depletion ( and B). Depression in osmotic potential may be positive in the sense of decreasing water influx and hence aluminum accumulation. The finding of Vitorello et al.,Citation1 that non-exchangeable aluminum uptake is inhibited in plasmolyzed cells may be supportive in this concept. Subsequently, NO3− efflux may decrease the osmotic potential since Ca depletion or Al treatment resulted in about 10% increase in NO3− efflux (data not shown) compared with control cells. However, NO3− efflux does play important role other than decreasing the osmotic potential. According to ref. Wendehenne et al.,Citation41 it has been regarded as an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco cells.
Taken together, it can be concluded that the common events shared by Ca depletion and aluminum toxicity lead to death of tobacco cells via damaging the membranes and decreasing the osmotic potential that inhibited water influx. Similar rates of NO3− efflux, per se would be considered a sign of hypersensitive cell death as reported earlier.Citation41 Nevertheless, whether this cell death is an apoptosis type needs further confirmation.
Materials and Methods
Tobacco cells, medium and culture conditions.
A non-chlorophylous tobacco cell line, SL (sometimes also ALT301), derived from Nicotiana tabacum L. cv. Samsun (Nakamura et al.Citation27) was used. The medium employed for cell growth was a modified version of Murashige-Skoog's (MS) medium (pH 5.0, after autoclaving; Yamamoto et al.Citation28,Citation29 Cells were maintained by transferring 2 mL of the cell suspension at 7-d intervals into 30 mL of fresh medium in a 100 mL Erlenmeyer flask.
Treatment with Al and Ca.
The treatment of tobacco cells with Al was performed as described previously (Yamamoto et al.Citation28). Tobacco cells in the logarithmic phase of growth on day 4 were washed three times with a simple Ca solution containing 3 mM CaCl2 and 3% sucrose (Ca medium) at pH 5.0, then finally suspended in the Ca medium (pH 4.5) at a cell density of 10 mg fresh weight mL−1. In the experiments where various Ca concentrations were studied, the washing solution was sucrose medium (3%) adjusted to pH 5.0 using Mes-BTP buffer (20 mM). Sucrose medium was autoclaved while Mes-BTP buffer and Ca solution were filter-sterilized. The cells were incubated on a rotary shaker operated at 100 rpm at 25°C for 18 h in darkness; after which the cells were harvested for analysis.
Assessment of growth capability.
After 18 hrs of treatment, an aliquot (5 mL) of the cell suspension was transferred into fresh MS medium devoid of any of the treatment (Ca or Al). After growth for 6 more days the fresh weight is re-determined. It represents the capability of the surviving cells to recommence growth.
Assessment of the loss of plasma membrane integrity.
Cell death accompanying the loss of membrane integrity was evaluated by a spectrophotometric assay of Evans blue stain retained by cells as described previously.Citation30 Briefly, cells were suspended in 2 mL of a 0.05% aqueous Evans blue solution and gently shaken for 15 min at room temperature. Then the cells were washed extensively, and the Evans blue trapped in the cells was released by suspension in 1% SDS solution and disrupting them in a sonicator. The sonicate was centrifuged, and the optical density of the supernatant was determined spectrophotometrically at 600 nm.
Protoplast preparation and determination of cell number.
Protoplasts derived from intact cells were used for the determination of cell number. For the preparation of protoplasts, 100 mg fresh weight of cells were treated with 2% cellulase ‘Onozuka’ R10 (Yakulto Pharma., Tokyo) and 0.5% pectyolase Y23 (Seishin Pharma., Tokyo) in 0.4 M mannitol, 0.025 M CaCl2, MES (pH 5.6) at 25°C, with rotation at 30 rpm, for 1.0 h. The protoplasts were sedimented by centrifugation and resuspended in 0.29 M mannitol, 0.125 M CaCl2, MES (pH 5.6) according to Potrykus and Shillito.Citation31 The number of protoplasts was determined with a hemacytometer under the microscope.
Measurements of osmotic potential.
Cultures of tobacco cells were filtered and the cells were homogenized in liquid nitrogen. The ground cells were collected immediately in Eppendorf tubes and centrifuged for 10 min at 12,000 rpm at 4°C. The clear supernatant (cell sap) was used to determine cellular osmotic potential. The freezing point Micro-osmometer (Model 210, Fiske Associates, Norwood, Massachuttes, USA) was used to measure the osmotic potential of cell sap as well as of the medium.
Assessment of NO3− efflux.
Ten mL of tobacco cell cultures were centrifuged for 5 min at 2000 g and the top 5 mL of the extracellular medium were transferred and filtered (0.45 µm) to remove any suspended cells. The NO3− concentration was determined using a colorimetric assay kit (Alexis Biochemicals) according to the procedure recommended by the supplier. To 80 µL of the extracellular medium, 10 µL of the enzyme cofactor was added, followed by 10 µL of nitrate reductase and the mixture was incubated for one hour at room temperature. The nitrite produced from nitrate reduction was converted into a purple azo compound by adding 50 µL of Griess reagent R1 (sulfanilamide) followed by 50 µL of Griess reagent R2 [N-(1-naphthyl)ethylenediamine]. After 10 min incubation, required to obtain optimal color development, absorbance was read at 540 nm using a photometer. Quantification of NO3− concentration was performed using a NO3− calibration curve (0–35 µM).
Statistical analysis.
Each experiment was repeated three times. All the presented values represent the means ± SE of triplicate results.
Figures and Tables
Figure 1 Fresh weight of tobacco cells (mg/cells in 10 mL culture) as influenced by successively increasing concentrations of calcium combined with or without 100 µM Al for 18 h (A). The inset (Aa) shows the impact of Ca concentrations ≤0.5 mM. Each point represents the mean value of three replicates ± SE of two independent experiments.
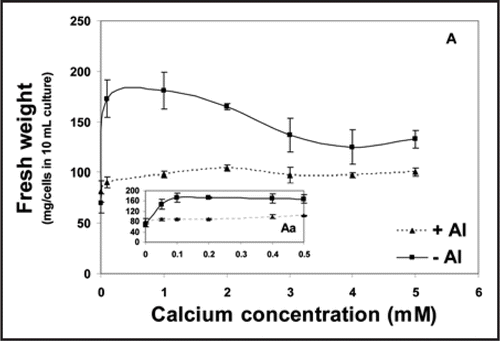
Figure 2 Growth capability of tobacco cells (mg/cells in 10 mL culture) subjected to successively increasing concentrations of calcium without Al (A) or combined with 100 µM Al (B). The insets (Aa) and (Bb) show the impact of only Ca concentrations ≤0.5 mM and when combined with Al, respectively. After being treated for 18 h, the cells were washed twice with Ca-sucrose medium (3 mM Ca, 3% sucrose, pH 5.8), then let grow in a modified MS medium for 6 more days. Thereafter, the fresh weight (mg/cells in 5 mL culture) was determined to represent the growth capability of only Ca-treated cells (A). In (B) the data of growth capability were plotted as percentages of fresh weight of Al-treated cells relative to those of Al free (control) ones. Each point represents the mean value of three replicates ± SE of two independent experiments.
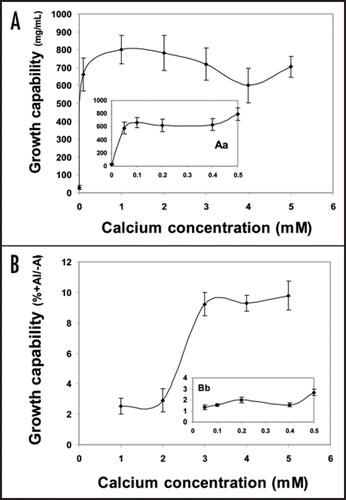
Figure 3 Fresh weight and water content (mg/106 cells) in Ca-depleted (- Ca), supplemented with 0.5 mM Ca (+ Ca) or Al-treated (Al) cultures for 18 h (A). This phenomenon has also been found to be consistent at 0.1 or 3 mM calcium. For the determination of cell density, protoplasts were isolated and counted using haemacytometer as described in ‘Materials and Methods’. (B) represents growth capability of variously treated tobacco cells. The cells were washed twice with sucrose solution (pH 5.8) and then resuspended in a modified MS medium and let grow for 6 days more. Thereafter, the fresh weight of 5 mL was determined. Each point represents the mean value of three replicates ± SE from two independent experiments.
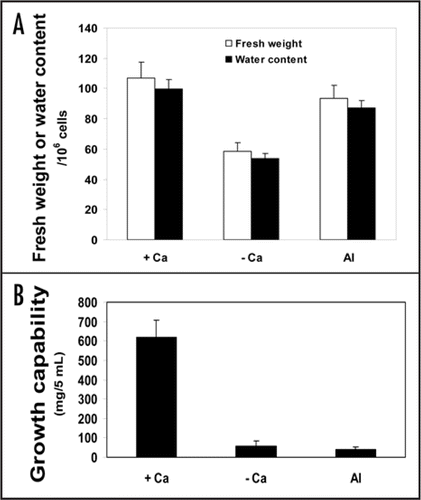
Figure 4 Membrane damage (Evans blue uptake) of tobacco cells in Ca-depleted (- Ca), supplemented with 0.5 mM Ca (+ Ca) or Al-treated (Al) cultures for 18 h. After treatment, the cells (10 mL aliquots at a cell density of 10 mg fresh weight mL−1) were collected and the integrity of the plasma membrane was determined by measurement of Evans blue retained as described in ‘Materials and Methods’. The data are presented as percentages relative to the value of Evans blue retained in tobacco cells boiled for 10 min (assumed to lose the plasma membrane integrity completely). Each point represents the mean value of three replicates ± SE from two independent experiments.
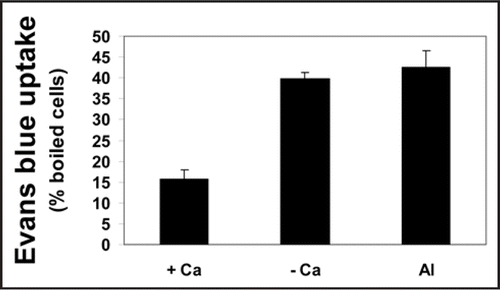
Figure 5 Osmotic potential of the Al-tolerant (ALT 301) and sensitive (SL) tobacco cell lines in Ca-depleted (- Ca), supplemented with 0.5 mM Ca (+ Ca) or Al-treated (Al) cultures for 18 h. Cells were filtered, homogenized in liquid nitrogen and then centrifuged. The clear supernatant (cell sap) was used to determine the osmotic potential using freezing point Micro-osmometer. Each point represents the mean value of three replicates ± SE from two independent experiments.
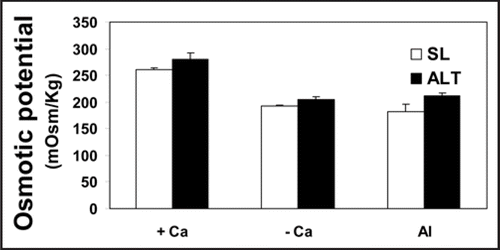
Figure 6 Kinetics of osmotic potential of tobacco cells (SL) in Ca-depleted (- Ca), supplemented with 0.5 mM Ca (+ Ca) or Al-treated (Al) cultures for 18 h (A). Cells were collected at 0, 3, 6 and 18 h after treatment and handled the same as in . (B) represents the fresh weight (mg/5 mL) at the above-mentioned time intervals. Each point represents the mean value of three replicates ± SE from two independent experiments.
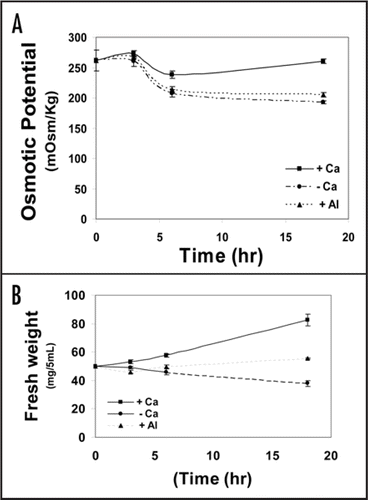
Table 1 Cell number, fresh weight and dry weight of tobacco suspension culture subjected for 18 h to Ca-supplementation with 0.5 mM (+Ca), Ca depletion (-Ca), or 100 µM Al in the presence of 0.5 mM Ca
Acknowledgements
R.A.B. sincerely thanks the JSPS (Japan) for financing his stay in RIB, Okayama University.
References
- Vitorello VA, Capaldi FR, Stefanuto VA. Recent advances in aluminum toxicity and resistance in higher plants. Braz J Plant Physiol 2005; 17:129 - 143
- Marienfeld S, Lehmann H, Stelzer R. Ultrastructural investigations and edx-analyses of Al-treated oat (Avena sativa) roots. Plant Soil 1995; 171:167 - 173
- Vitorello VA, Haug A. Short-term aluminum uptake by tobacco cells: Growth dependence and evidence for internalization in a discrete peripheral region. Physiol Plant 1996; 97:536 - 544
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. International Review of Cytology 2000; 200:1 - 46
- Matsumoto H, Yamamoto Y, Rama Devi S. Aluminum toxicity in acid soils—Plant response to aluminum. Metals in Environment: Analysis by Biodiversity M.N.V. Prasad 2001; New York. Basel Marcel Dekker Inc 289 - 319
- Takabatake R, Shimon T. Inhibition of electrogenesis by aluminum in characean cells. Plant Cell Physiol 1997; 38:1264 - 1271
- Zhang GC, Slaski JJ, Archambault DJ, Taylor GJ. Alteration of plasma membrane lipids in aluminum resistant and aluminum sensitive wheat genotypes in response to aluminum stress. Physiol Plant 1997; 99:302 - 308
- Taylor GJ, McDonald Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol 2000; 123:987 - 996
- Ishikawa S, Wagatsuma T. Plasma membrane permeability of root-tip cells following temporary exposure to Al ions is a rapid measure of Al tolerance among plant species. Plant Cell Physiol 1998; 39:516 - 525
- Zhao XL, Sucoff E, Stadelmann EJ. Al3+ and Ca2+ alteration of membrane permeability of Quercus rubra root cortex cells. Plant Physiol 1987; 83:159 - 162
- Vierstra R, Haug A. The effects of Al3+ on the physical properties of membrane lipids in Thermoplasma acidphilum. Biochem Biophys Res Commun 1978; 84:138 - 144
- Liu K, Luan S. Internal aluminum block of plant inward K+ channels. Plant Cell 2001; 13:1453 - 1465
- Yamamoto Y, Kobayashi Y, Rama Devi S, Rikiishi S, Matsumoto H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiology 2002; 128:63 - 72
- Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H. Oxidative stress triggered by aluminum in plant roots. Plant Soil 2003; 255:239 - 243
- Exley C. The pro-oxidant activity of aluminum. Free Radic Biol Med 2004; 36:380 - 387
- Foy CD, Chaney RC, White MC. The Physiology of metal toxicity in plants. Ann. Rev. Plant Physiol 1978; 29:506 - 511
- Ryan PR, Kochian LV. Interaction between aluminum toxicity and calcium uptake at the root apex in near-isogenic lines of wheat (Triticum aestivum L.) differing in aluminum tolerance. Plant Physiol 1993; 102:975 - 982
- Rengel Z, Zhang WH. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol 2003; 159:295 - 314
- Kinraide TB, Parker DR. Cation amelioration of aluminum toxicity in wheat. Plant Physiol 1987; 83:546 - 551
- Haug A, Vitorello V. Aluminium coordination to calmodulin: Thermodynamic and kinetic aspects. Coord Chem Rev 1996; 149:113 - 124
- Jones DL, Shaff JE, Kochian LV. Effect of aluminum on calcium homeostasis and IP3 mediated signal-transduction in Triticum aestivum and Nicotiana plumbaginifolia. Plant Physiol 1995; 108:41
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of Signaling. The Plant Cell 2002; 401 - 417
- Rengel Z. Uptake of aluminum by plant cells. New Phytol 1996; 137:389 - 406
- Lindberg S, Strid H. Aluminum induces rapid changes in cytoslic pH and free calcium and potassium concentrations in root protoplasts of wheat (Triticum aestivum). Physc Plant 1997; 99:405 - 414
- Matsumoto H, Senoo Y, Kasai M, Maeshma M. Response of the plant root to aluminum stress: Analysis of the inhibition of the root elongation and changes in membrane function. J Plant Res 1996; 109:99 - 105
- Takano M, Shimmen T. Effect of aluminum on plasma membrane as revealed by analysis of alkaline band formation in internodal cells of Chara corallina. Cell structure and function 1999; 24:131 - 137
- Nakamura C, Telgen HV, Mennes AM, Ono H, Libbenga KR. Correlation between auxin resistance and the lack of amembrane-bound auxin binding protein and a root-specific peroxidase in Nicotiana tabacum. Plant Physiol 1988; 88:845 - 849
- Yamamoto Y, Rikiishi S, Chang YC, Ono K, Kansai M, Matsumoto H. Quantitative estimation of aluminum toxicity in cultured tobacco cells: Correlation between aluminum uptake and growth inhibition. Plant Cell Physiology 1994; 35:575 - 583
- Yamamoto Y, Masamoto K, Hachiya A, Rikiishi S, Yamaguchi Y, Matsumoto H. Aluminum tolerance acquired during phosphate starvation in cultured tobacco cells. Plant Physiol 1996; 112:217 - 227
- Ikegawa H, Yamamoto Y, Matsumoto H. Cell death caused by a combination of aluminum and iron in cultured tobacco cells. Physiol Plant 1998; 104:474 - 478
- Potrykus I, Shillito RD. Weissbach A, Weissbach H. Protoplasts: Isolation, culture, plant regeneration. Methods for plant Molecular Biology 1988; San Diego Academic Press Inc 355 - 383
- Huang JW, Shaff JE, Grunes DL, Kochian LV. Aluminum effects on the kinetics of calcium fluxes at the root apex of aluminum-tolerant and aluminum-sensitive wheat cultivars. Plant Physiol 1992; 98:230 - 237
- Kinraide TB, Ryan PR, Kochian LV. Interactive effects of Al3+, H+ and other cations in root elongation considered in terms of cell-surface electrical potential. Plant Physiol 1992; 90:1461 - 1468
- Ahn SJ, Sivaguru M, Osawa H, Chung GC, Matsumoto H. Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potential in squash root. Plant Physiol 2001; 126:1381 - 1390
- Ikegawa H, Yamamoto Y, Matsumoto H. Responses to Aluminum of suspension-cultured tobacco cells in a simple calcium solution. Soil Sci Plant Nutr 2000; 46:503 - 514
- Kauss H. Some aspects of calcium-dependent regulation in plant metabolism. Ann Rev Plant Physiol 1987; 88:418 - 423
- Subbaiah CC, Sachs MM. Altered pattern of sucrose synthase phosphorylation and localization preced callose induction and root tip death in anoxic maize seedlings. Plant Physiol 2001; 125:585 - 594
- Yim KO, Bradford KJ. Callose deposition is responsible for apoplastic semipermeability of the endosperm envelope of muskelon seeds. Plant Physiol 1998; 118:83 - 90
- Sivaguru M, Fujiwara T, Samaj J, Balsuska F, Yang Z, Osawa H, Maeda T, Mori T, Folkmann D, Matsumoto H. Aluminum-induced 1, 3-β-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol 2000; 124:991 - 1005
- Sivaguru M, Horst WT, Eticha D, Matsumoto H. Aluminum inhibits apoplastic flow of high-molecular weight solutes in root apices of Zea mays L. J Plant Nutr Soil Sci 2006; 169:679 - 690
- Wendehenne D, Lamotte O, Frachissee JM, Barbier Brygoo H, Pugin A. Nitrate efflux is essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. The Plant Cell 2002; 14:1937 - 1951