Abstract
The conserved eukaryotic protein SGT1 (Suppressor of G2 allele of skp1) participates in diverse physiological processes such as cell cycle progression in yeast, plant immunity against pathogens and plant hormone signalling. Recent genetic and biochemical studies suggest that SGT1 functions as a novel co-chaperone for cytosolic/nuclear HSP90 and HSP70 molecular chaperones in the folding and maturation of substrate proteins. Since proteins containing the leucine-rich repeat (LRR) protein-protein interaction motif are overrepresented in SGT1-dependent phenomena, we consider whether LRR-containing proteins are preferential substrates of an SGT1/HSP70/HSP90 complex. Such a chaperone organisation is reminiscent of the HOP/HSP70/HSP90 machinery which controls maturation and activation of glucocorticoid receptors in animals. Drawing on this parallel, we discuss the possible contribution of an SGT1-chaperone complex in the folding and maturation of LRR-containing proteins and its evolutionary consequences for the emergence of novel LRR interaction surfaces.
The proper folding and maturation of proteins is essential for cell viability during de novo protein synthesis, translocation, complex assembly or under denaturing stress conditions. A complex machinery composed of molecular chaperones (heat-shock proteins, HSPs) and their modulators known as co-chaperones, catalyzes these protein folding events.Citation1,Citation2 In animals, defects in the chaperone machinery is implicated in an increasing number of diseases such as cancers, susceptibility to viruses, neurodegenerative disease and cystic fibrosis, and thus it has become a major pharmacological target.Citation3,Citation4 In plants, molecular genetic studies have identified chaperones and co-chaperones as components of various physiological responses and are now starting to yield important information on how chaperones work. Notably, processes in plant innate immunity rely on the HSP70 and HSP90Citation5–Citation7 chaperones as well as two recently characterised co-chaperones, RAR1 (required for Mla12 resistance) and SGT1 (suppressor of G2 allele of skp1).Citation8–Citation11
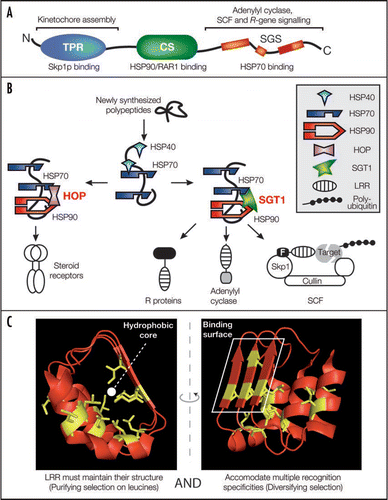
SGT1 is a highly conserved and essential co-chaperone in eukaryotes and is organized into three structural domains: a tetratricopeptide repeat (TPR), a CHORD/SGT1 (CS) and an SGT1-specific (SGS) domain (). SGT1 is involved in a number of apparently unrelated physiological responses ranging from cell cycle progression and adenylyl cyclase activity in yeast to plant immunity against pathogens, heat shock tolerance and plant hormone (auxin and jasmonic acid) signalling.Citation7–Citation9,Citation12,Citation13 Because the SGT1 TPR domain is able to interact with Skp1, SGT1 was initially believed to be a component of SCF (Skp1/Cullin/F-box) E3 ubiquitin ligases that are important for auxin/JA signalling in plants and cell cycle progression in yeast.Citation13,Citation14 However, mutagenesis of SGT1 revealed that the TPR domain is dispensable for plant immunity and auxin signalling.Citation15 Also, SGT1-Skp1 interaction was not observed in Arabidopsis.Citation13 More relevant to SGT1 functions appear to be the CS and SGS domains.Citation16 The former is necessary and sufficient for RAR1 and HSP90 binding. The latter is the most conserved of all SGT1 domains and the site of numerous disabling mutations.Citation14,Citation16,Citation17
We recently demonstrated that Arabidopsis SGT1 interacts stably through its SGS domain with cytosolic/nuclear HSP70 chaperones.Citation7 The SGS domain was both necessary and sufficient for HSP70 binding and mutations affecting SGT1-HSP70 interaction compromised JA/auxin signalling and immune responses. An independent in vitro study also found interaction between human SGT1 and HSP70.Citation18 The finding that SGT1 protein interacts directly with two chaperones (HSP90/70) and one co-chaperone (RAR1) reinforces the notion that SGT1 behaves as a co-chaperone, nucleating a larger chaperone complex that is essential for eukaryotic physiology. A future challenge will be to dissect the chaperone network at the molecular and subcellular levels. In plant cells, SGT1 localization appears to be highly dynamic with conditional nuclear localizationCitation7 and its association with HSP90 was recently shown to be modulated in vitro by RAR1.Citation16
A co-chaperone function suits SGT1 diverse physiological roles better than a specific contribution to SCF ubiquitin E3 ligases. Because SGT1 does not affect HSP90 ATPase activity, SGT1 was proposed rather as a scaffold protein.Citation16,Citation19 In the light of our findings and earlier studies,Citation20 SGT1 is reminiscent of HOP (Hsp70/Hsp90 organizing protein) which links HSP90 and HSP70 activities and mediates optimal substrate channelling between the two chaperones ().Citation21 While the contribution of the HSP70/HOP/HSP90 to the maturation of glucocorticoid receptors is well established,Citation21 direct substrates of an HSP70/SGT1/HSP90 complex remain elusive.
It is interesting that SGT1 appears to share a functional link with leucine-rich repeat- (LRR) containing proteins although LRR domains are not so widespread in eukaryotes. For example, plant SGT1 affects the activities of the SCFTIR1 and SCFCOI1 E3 ligase complexes whose F-box proteins contain LRRs.Citation13 Moreover, plant intracellular immune receptors comprise a large group of LRR proteins that recruit SGT1.Citation8,Citation9 LRRs are also found in yeast adenylyl cyclase Cyr1p and the F-box protein Grr1p which is required for SGT1-dependent cyclin destruction during G1/S transition.Citation12,Citation14 Yeast 2-hybrid interaction assays also revealed that yeast and plant SGT1 tend to associate directly or indirectly with LRR proteins.Citation12,Citation22,Citation23 We speculate that SGT1 bridges the HSP90-HSC70 chaperone machinery with LRR proteins during complex maturation and/or activation. The only other structural motif linked to SGT1 are WD40 domains found in yeast Cdc4p F-box protein and SGT1 interactors identified in yeast two-hybrid screens.Citation12
What mechanisms underlie a preferential SGT1-LRR interaction? HSP70/SGT1/HSP90 may have co-evolved to assist specifically in folding and maturation of LRR proteins. Alternatively, LRR structures may have an intrinsically greater need for chaperoning activity to fold compared to other motifs. These two scenarios are not mutually exclusive. The LRR domain contains multiple 20 to 29 amino acid repeats, forming an α/β horseshoe fold.Citation24 Each repeat is rich in hydrophobic leucine/isoleucine residues which are buried inside the structure and form the structural backbone of the motif (, left). Such residues are under strong purifying selection to preserve structure. These hydrophobic residues would render the LRR a possible HSP70 substrate.Citation25 By contrast, hydrophilic solvent- exposed residues of the β strands build a surface which confers ligand recognition specificity of the LRRs (). In many plant immune receptors for instance, these residues are under diversifying selection that is likely to favour the emergence of novel pathogen recognition specificities in response to pathogen evolution.Citation26 The LRR domain of such a protein has to survive such antagonist selection forces and yet remain functional. Under strong selection pressure, LRR proteins might need to accommodate less stable LRRs because their recognition specificities are advantageous. This could be the point at which LRRs benefit most from a chaperoning machinery such as the HSP90/SGT1/HSP70 complex. This picture is reminiscent of the genetic buffering that HSP90 exerts on many traits to mask mutations that would normally be deleterious to protein folding and/or function, as revealed in Drosophila and Arabidopsis.Citation27 It will be interesting to test whether the HSP90/SGT1/HSP70 complex acts as a buffer for genetic variation, favouring the emergence of novel LRR recognition surfaces in, for example, highly co-evolved plant-pathogen interactions.Citation28,Citation29
Figures and Tables
Figure 1 Model for SGT1/chaperone complex functions in the folding of LRR-containing proteins. (A) The structural domains of SGT1, their sites of action (above) and respective binding partners (below) are shown. N- and C-termini are indicated. TPR, tetratricopeptide repeat; CS, CHORD/SGT1; SGS, SGT1-specific. (B) Conceptual analogy between steroid receptor folding by the HOP/chaperone machinery and LRR protein folding by the SGT1/chaperone machinery. LRR motifs are overrepresented in processes requiring SGT1 such as plant immune receptor signalling, yeast adenylyl cyclase activity and plant or yeast SCF (Skp1/Cullin/F-box) E3 ubiquitin ligase activities. (C) Opposite forces drive LRR evolution. Structure of LRRs 16 to 18 of the F-box auxin receptor TIR1 is displayed as an illustration of the LRR folds.Citation30 Leucine/isoleucine residues (side chain displayed in yellow) are under strong purifying selection and build the hydrophobic LRR backbone (Left). By contrast, solvent-exposed residues of the β-strands define a polymorphic and hydrophilic binding surface conferring substrate specificity to the LRR (Right) and are often under diversifying selection.
References
- Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 2004; 5:781 - 791
- Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci 2003; 28:541 - 547
- Powers MV, Workman P. Inhibitors of the heat shock response: biology and pharmacology. FEBS letters 2007; 581:3758 - 3769
- Barral JM, Broadley SA, Schaffar G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Sem Cell & Dev Biol 2004; 15:17 - 29
- Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, Dangl JL. Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J 2003; 22:5679 - 5689
- Takahashi A, Casais C, Ichimura K, Shirasu K. HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc Nat Acad Sci USA 2003; 100:11777 - 11782
- Noël LD, Cagna G, Stuttmann J, Wirthmuller L, Betsuyaku S, Witte CP, Bhat R, Pochon N, Colby T, Parker JE. Interaction between SGT1 and Cytosolic/Nuclear HSC70 Chaperones Regulates Arabidopsis Immune Responses. Plant Cell 2007; 19:4061 - 4076
- Austin MJ, Muskett P, Kahn K, Feys BJ, Jones JD, Parker JE. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 2002; 295:2077 - 2080
- Azevedo C, Sadanandom A, Kitagawa K, Freialdenhoven A, Shirasu K, Schulze-Lefert P. The RAR1 interactor SGT1, an essential component of R gene- triggered disease resistance. Science 2002; 295:2073 - 2076
- Muskett PR, Kahn K, Austin MJ, Moisan LJ, Sadanandom A, Shirasu K, Jones JDG, Parker JE. Arabidopsis RAR1 exerts rate-limiting control of R gene- mediated defenses against multiple pathogens. Plant Cell 2002; 14:979 - 992
- Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, Dangl JL. RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 2002; 14:1005 - 1015
- Dubacq C, Guerois R, Courbeyrette R, Kitagawa K, Mann C. Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Euk Cell 2002; 1:568 - 582
- Gray WM, Muskett PR, Chuang HW, Parker JE. Arabidopsis SGT1b is required for SCFTIR1-mediated auxin response. Plant Cell 2003; 15:1310 - 1319
- Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Heiter P. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a subunit of the SCF ubiquitin ligase complex. Mol Cell 1999; 4:21 - 33
- Azevedo C, Betsuyaku S, Peart J, Takahashi A, Noël L, Sadanandom A, Casais C, Parker J, Shirasu K. Role of SGT1 in resistance protein accumulation in plant immunity. Embo J 2006; 25:2007 - 2016
- Botër M, Amigues B, Peart J, Breuer C, Kadota Y, Casais C, Moore G, Kleanthous C, Ochsenbein F, Shirasu K, Guérois R. Structural and Functional Analysis of SGT1 Reveals That Its Interaction with HSP90 Is Required for the Accumulation of Rx, an R Protein Involved in Plant Immunity. Plant Cell 2007; 19:3791 - 3804
- Bansal PK, Abdulle R, Kitagawa K. Sgt1 associates with Hsp90: an initial step of assembly of the core kinetochore complex. Mol Cell Biol 2004; 24:8069 - 8079
- Spiechowicz M, Zylicz A, Bieganowski P, Kuznicki J, Filipek A. Hsp70 is a new target of Sgt1—an interaction modulated by S100A6. Biochem Biophys Res Commun 2007; 357:1148 - 1153
- Catlett MG, Kaplan KB. Sgt1p is a unique co-chaperone that acts as a client adaptor to link Hsp90 to Skp1p. J Biol Chem 2006; 281:33739 - 33748
- Schulze Lefert P. Plant immunity: The origami of receptor activation. Curr Biol 2004; 14:22 - 24
- Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem 2004; 40:41 - 58
- Bieri S, Mauch S, Shen QH, Peart J, Devoto A, Casais C, Ceron F, Schulze S, Steinbiss HH, Shirasu K, Schulze-Lefert P. RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. Plant Cell 2004; 16:3480 - 3495
- Leister RT, Dahlbeck D, Day B, Li Y, Chesnokova O, Staskawicz BJ. Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana. Plant Cell 2005; 17:1268 - 1278
- Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Structural Biol 2001; 11:725 - 732
- Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J 1997; 16:1501 - 1507
- Bakker EG, Toomajian C, Kreitman M, Bergelson J. A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 2006; 18:1803 - 1818
- Rutherford SL. Between genotype and phenotype: protein chaperones and evolvability. Nat Rev Genet 2003; 4:263 - 274
- Dodds PN, Lawrence GJ, Catanzariti AM, Teh T, Wang CI, Ayliffe MA, Kobe B, Ellis JG. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc Natl Acad Sci USA 2006; 103:8888 - 8893
- Wang CI, Guncar G, Forwood JK, Teh T, Catanzariti AM, Lawrence GJ, Loughlin FE, Mackay JP, Schirra HJ, Anderson PA, Ellis JG, Dodds PN, Kobe B. Crystal structures of flax rust avirulence proteins AvrL567-A and -D reveal details of the structural basis for flax disease resistance specificity. Plant Cell 2007; 19:2898 - 2912
- Tan X, Calderon Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007; 446:640 - 645