Abstract
During the last decade, growing evidence has arisen referring the importance of the proper regulation of plant polyamine metabolism in the response to stress conditions. Being the activation of signaling pathways, the stabilization of anionic molecules and prevention of their degradation, as well as the free radical scavenger properties of polyamines some possible mechanisms exerted by these amines. Accumulation of polyamines (putrescine, spermidine and spermine) has been associated to plant tolerance to a wide array of environmental stresses. The synthesis of spermidine and spermine is mediated by aminopropyltransferases (spermidine and spermine synthases) which constitute a class of widely distributed enzymes that use decarboxylated S-adenosylmethionine as an aminopropyl donor, and putrescine or spermidine as an amino acceptor. We recently reported the effect of salt stress on the expression of aminopropyltransferase genes in maize seedlings. Our data revealed a time and NaCl dependent regulation of the Zmspds2 and Zmspds1 genes, possibly mediated by abscisic acid, since these genes were regulated at the transcriptional level by this plant hormone. In this addendum, we show that the Zmspds2 gene initially classified as spermidine synthase might encode a spermine synthase based on an in silico analysis. This is discussed in terms of protein homologies and specific amino acid substitutions between aminopropyltransferase enzymes.
Aminopropyltransferases such as spermidine synthase (SPDS, EC 2.5.1.16) and spermine synthase (SPMS, EC 2.5.1.22) belong to a class of widely distributed enzymes that use decarboxylated S-adenosyl-methionine (dcSAM) as an aminopropyl donor, and putrescine or spermidine as an amino acceptor to form in that order, spermidine or spermine. The dcSAM required for this reaction is derived from S-adenosyl-methionine by the action of S-adenosyl-methionine decarboxylase. Putrescine, spermidine and spermine, the common polyamines in plants, are small aliphatic amines which have been implicated in a wide range of plant growth and developmental processes including cell division, embryogenesis, morphogenesis, fruit development and ripening, leaf senescence and response to environmental stresses.Citation1–Citation4
Zmspds2 Maize Gene
In a previous work, we isolated an aminopropyltransferase cDNA sequence from maize (GenBank AY730048),Citation5 which showed high sequence identity with the rice SPDS2 enzyme (81.9%, GenBank AB098063),Citation6 and considerably less identity with the dicot SPDSs (ca. 50%) and ACL5 (ca. 20%) enzymes. For this reason, this sequence was named Zmspds2. Using a detached maize leaf system, we first reported the transcriptional regulation of Zmspds2 gene under salt stress and the appearance of two Zmspds2 splicing variants (Zmspds2A y Zmspds2B), upregulated in a period of hours in this response.Citation5 The Zmspds2A variant is originated by removal of introns and setting exons together, while in the shorter Zmspds2B variant, in addition to the introns the second exon of the ORF is removed by alternative splicing.Citation5
In a further research, we evaluated if longer salt stress treatments (days) had an effect over the regulation of Zmspds2 gene in leaves of maize seedlings grown under hidroponically conditions.Citation7 In agreement with our previous findings, longer periods of salt stress still induced the expression of both Zmspds2 (A and B) splicing variants, been the later only expressed in response to NaCl. Our data showed a time and NaCl dependent regulation of the Zmspds2 gene, suggesting that this gene might be regulated in response to a hyperosmotic stress.Citation7
Recently, we conducted in silico analysis of the Zmspds2 gene considering protein sequence homologies as well as conserved amino acid substitutions respecting spermidine synthases, in which unique amino acid residues have been reported to be involved in the formation of a putrescine binding-cavity.Citation8,Citation9 Based on previous reportsCitation6,Citation8–Citation10 and our in silico evidences (shown bellow), it is revealed that the Zmspds2 gene might encode a spermine synthase rather than a spermidine synthase. In this sense, further in the text we will refer the Zmpsds2 gene as Zmspms.
A phylogenetic tree including the SPMS proteins from Arabidopsis, apple, grape, rice and poplar, and the SPDSs from different plant species is shown (). In this Figure, we observed a clustering of the ZmSPMS (previous ZmSPDS2) and OsSPDS2 proteins within the well characterized spermine synthases from Arabidopsis (AtSPMS)Citation10,Citation11 and apple (MdSPMS),Citation9 and the ortologs of these genes in grape and poplar (GENOSCOPE, Grape Genome Browser; JGI, Populus trichocarpa project). Functionality of the AtSPMS and MdSPMS enzymes has been accurately determined by complementation analysis using spms defective yeast mutants and Arabidopsis acl5 mutants.Citation9–Citation11
Comparison analysis of the maize ZmSPMS protein with the described SPMSs showed the following identities: 69.5% with poplar PtSPMS (GENOSCOPE), 69.4% with grape VvSPMS (JGI), 68.2% with Arabidopsis AtSPMS (NP_568785), and 66.7% with the Malus domestica MdSPMS (BAE19758), whereas with the SPDS proteins of Arabidopsis, AtSPDS1 (CAB61614) and AtSPDS2 (CAB61615) shares 57.8 and 55.3%, respectively. Notably, monocot ZmSPMS and OsSPDS2 proteins appear to be are larger than dicots SPMS through the carboxi-termini of the protein, possibly a characteristic feature of monocot SPMS enzymes ().
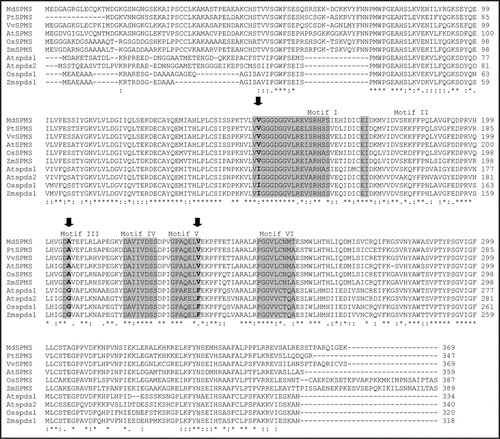
At the protein level, it has been well documented that few amino acids are unique and conserved in the SPDS's enzymes and contribute to the formation of the putrescine binding cavity.Citation8,Citation9 The ZmSPMS protein could be distinguished from the SPDS's by specific amino acid changes: Phe replaced by Val-234, Ile by Val-148 and Gly by Ala-204 (). These changes were also conserved in the Arabidopsis and apple SPMS's as well as in the rice, grape and poplar ortologs.Citation9
The importance of aminopropyltransferase genes under plant abiotic stress conditions is increasing steeply.Citation5,Citation7,Citation12,Citation13 In this sense, our evidences under salt stress conditions point that the Zmspms gene (previous Zmspds2)Citation5,Citation7 undergoes specific regulation processes, including alterative splicing events, possibly leading to an important regulation of polyamine homeostasis under stress conditions.
Figures and Tables
Figure 1 Phylogenetic tree representing the SPMS's and SPDS's from different plants. SPMS: Malus domestica (MdSPMS), Populus trichocarpa (PtSPMS), Vitis vinifera (VvSPMS), Arabidopsis thaliana (AtSPMS) and Oryza sativa (OsSPDS2); SPDS: Arabidopsis thaliana (AtSPDS1 and AtSPDS2), Oryza sativa (OsSPDS1), Populus trichocarpa (PtSPDS1 and PtSPDS2) and Malus domestica (MdSPDS1 and MdSPDS2); ACL5: Arabidopsis (AtACL5); were created by the neighbor-joining method using the MEGA version 3.1 program.Citation14 The distance scale represents evolutionary distance expressed as the number of substitutions per residue.
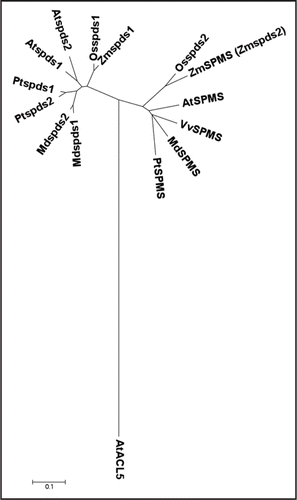
Figure 2 Multiple amino acid alignment as derived by maximal homology of ZmSPMS protein (previous ZmSPDS2) with the corresponding protein sequences of other plant SPMSs: MdSPMS, Malus domestica (BAE19758), PtSPMS, Populus trichocarpa (JGI), VvSPMS, Vitis vinifera (GENOSCOPE), AtSPMS, Arabidopsis thaliana (NP_568785), OsSPDS2, Oryza sativa (BAD54209); and SPDSs: AtSPDS1, Arabidopsis thaliana (CAB61614), AtSPDS2, Arabidopsis thaliana (CAB61615), OsSPDS1, Oryza sativa (NP_912671). Identical residues (asterisks) in the ten proteins and conserved amino acid substitutions (dots) are indicated. The proposed binding sites for SAM and dcSAM (motifs I to VI) are shadowed. The unique and conserved amino acids in the SPDS's that contribute to the formation of the putrescine binding cavity are indicated with an arrow.
Addendum to:
References
- Bouchereau A, Aziz A, Larher F, Martin Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci 1999; 140:103 - 125
- Kaur Sawhney R, Tiburcio AF, Altabella T, Galston A. Polyamines in plants: an overview. J Cell Mol Biol 2003; 2:1 - 12
- Liu JH, Kitashiba H, Wang J, Ban Y, Moriguchi T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol 2007; 24:117 - 126
- Pang XM, Zhang ZY, Wen XP, Ban Y, Moriguchi T. Polyamines, All-purpose players in response to environment stresses in plants. Plant Stress 2007; 1:173 - 188
- Rodríguez Kessler M, Alpuche Solís AG, Ruiz OA, Jiménez Bremont JF. Effect of salt-stress on the regulation of maize (Zea mays L) genes involved in polyamine biosynthesis. Plant Growth Regul 2006; 48:175 - 185
- Imai R, Ali A, Pramanik HR, Nakaminami K, Sentoku N, Kato H. A distinctive class of spermidine synthase is involved in chilling response in rice. J Plant Physiol 2004; 161:883 - 886
- Jiménez Bremont JF, Ruiz OA, Rodríguez Kessler M. Modulation of spermidine and spermine levels in maize seedlings subjected to long-term salt. Plant Physiol Biochem 2007; 45:812 - 821
- Korolev S, Ikeguchi Y, Skarina T, Beasley S, Arrowsmith C, Edwards A, Joachimiak A, Pegg AE, Savchenko A. The crystal structure of spermidine synthase with a multisubstrate adduct inhibitor. Nat Struct Biol 2002; 9:27 - 31
- Kitashiba H, Hao YJ, Honda C, Moriguchi T. Two types of spermine synthase gene: MdACL5 and MdSPMS are differentially involved in apple fruit development and cell growth. Gene 2005; 361:101 - 111
- Hanzawa Y, Imai A, Michael AJ, Komeda Y, Takahashi T. Characterization of the spermidine synthase-related gene family in Arabidopsis thalina. FEBS Letts 2002; 527:176 - 180
- Panicot M, Minguet EG, Ferrando A, Alcazar R, Blazquez MA, Carbonell J, Altabella T, Koncz C, Tiburcio AF. A polyamine metabolon involving aminopropyl transferase complexes in Arabidopsis. Plant Cell 2002; 14:2539 - 2551
- Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and upregulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 2004; 45:712 - 722
- Liu JH, Inoue H, Moriguchi T. Salt stress-mediated changes in free polyamine titers and expression of genes responsible for polyamine biosynthesis of apple in vitro shoots. Environm Exp Bot 2008; 62:28 - 35
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 2004; 5:150 - 163