Abstract
In a recent paper in Planta, we combined novel confocal reflectance imaging of intracellular reactive oxygen species (ROS) with inhibition-of-growth experiments to show that ROS help to direct polarized growth in brown algal zygotes. Using confocal fluorescence imaging of intracellular Ca2+ distributions, we were also able to show an interaction between ROS and Ca2+ signalling. The modulation of intracellular Ca2+ signals by reactive oxygen species (ROS) is a common motif in many plant and algal systems, but our Planta paper is its first demonstration during early development. We explain here how our findings complement a number of recent studies on polarized growth in plant and algal systems.
In many multicellular organisms an initial asymmetric division of the zygote is a critical event during early development. The acquisition and expression of a zygotic axis of polarity ensures that the first cell division will give rise to two daughter cells with distinct cellular identities. This polarity is key to the assignation of different cell fates to different early embryonic cells and hence to the generation of correct multicellular patterning. However, the principles underlying the organization of polarity acquisition remain elusive. Different model organisms, such as the fruit fly Drosophila melanogaster, the flowering plant Arabidopsis thaliana and the brown alga Fucus serratus, have been used to probe different aspects of zygotic polarization and, taken together, the evidence shows that a regulatory network of genes, proteins and small signaling molecules (such as Ca2+ ions) act to specify the direction of polarization.
One crucial insight has been to show that axes of polarity are defined by ionic and molecular gradients. These gradients are often expressed by a number of cellular components, with protein gradients being the best studied. The most famous of these is undoubtedly found in animals—cell specification in developing D. melanogaster embryos is determined by the anterior-posterior body axis.Citation1 This is derived from the initial axis of zygote polarity which is, in turn, defined by the famous bicoid transcription factor gradient set up during oögenesis in response to maternal organizers.Citation2 Similar protein gradients have also been shown to direct polarization in flowering plants. In Arabidopsis thaliana, for example, polarized distribution of the PIN auxin transporterCitation3 helps to specify an apical-basal axis during embryogenesis,Citation4 which then determines the shoot-root axis of the adult plant.
Unfortunately, fewer data are available about the role played by non-protein, small signaling molecule gradients. Many of the model organisms which allow genetic dissection of protein regulatory networks are poor models in which to perform the physiological studies needed to follow small signaling molecules. Flowering plant embryos, for example, are concealed inside ovules which supply maternal cues to polarization, making real-time visualization of small molecule gradients extremely difficult.
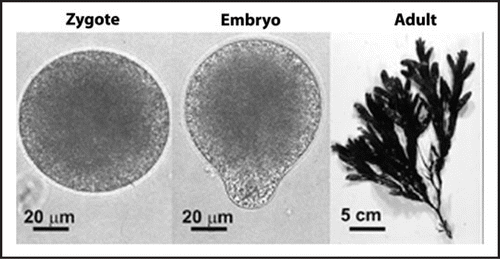
To circumvent this problem, small molecule gradients are often studied in brown algae.Citation5 Gametes of the marine brown algae Fucus and Pelvetia, for example, are large, easy to obtain and externally fertilized, making them particularly suitable for cellular imaging studies. Furthermore, brown algal zygotes polarize in response to external cues, most notably the direction of incident light,Citation6,Citation7 allowing for precise experimental control of the direction and timing of polar axis acquisition. Such studies have revealed that zygote polarity is established in three stages; (a) the generation of asymmetries along the incident light gradient a few hours after fertilization (axis formation),Citation6,Citation7 (b) the stabilization of the asymmetries around 6–10 hours after fertilization (axis fixation)Citation8 and, following this photopolarization process, (c) the separation of these asymmetries into two different cells—the rhizoid and the thallus cell ()—around 12–18 hours after fertilization (germination and cell division). This polar axis defines the orientation of zygotic growth, the plane of the first cell division, the distinct fates of the asymmetric daughter cells and the polarity of the organism, with subsequent cell divisions occuring in a highly ordered spatial and temporal pattern.Citation5
What have brown algal zygotes revealed about embryogenic small molecule gradients? The most striking findings have shown that the axis of polarity in brown algal zygotes is defined, at least in part, by an intracellular Ca2+ gradient which can be visualized as early as 1 hour after exposure to unilateral blue light during the photosensitive periodCitation9,Citation10 and which persists through rhizoid germination and elongation.Citation9
In our Planta paper, we decided to look at how this central Ca2+ gradient was maintained and to examine what controls existed to ensure its robustness. To do this, we took our cue from earlier work by ourselves and others on non-embryonic cells which display polarized growth, most notably root hairsCitation11 and pollen tubes.Citation12 In both of these cell types, it has been shown that Ca2+ influx can be stimulated by reactive oxygen species (ROS). ROS are an inescapable consequence of aerobic metabolism in eukaryotes, but may also be generated in a controlled manner by intracellular processes and have been ascribed a number of regulatory functions. One of the main sources of such regulatory ROS are the plasma membrane localized NADPH oxidases. These convert molecular oxygen to superoxide anions (O2−) which may, in turn, dismute to H2O2 or hydroxyl radicals (OH).
Given that the ROS-Ca2+ link is a well-established and evolutionarily conserved signaling motif,Citation13,Citation14 it seemed sensible to investigate whether a similar ROS-Ca2+interdependence might exist in F. serratus zygotes. The problems were largely technical; fluorescent and absorbance dyes which report ROS are ‘one-shot’ non-equilibrium dyes. This means that they report all the ROS production which has occurred since the dyes were introduced, rather than providing a snapshot of ROS generation during the second or so over which images were acquired. The non-equilibrium nature of the dyes meant that stringent control experiments were needed if sensible inferences were to be made about the patterns of dye intensities. We therefore used two dyes, the fluorescent H2O2 and OH-sensitive chloromethyl-2′,7′-dichlorofluorescein (CM-DCF) and the absorbant O2−-sensitive nitroblue tetrazolium (NBT).Citation15
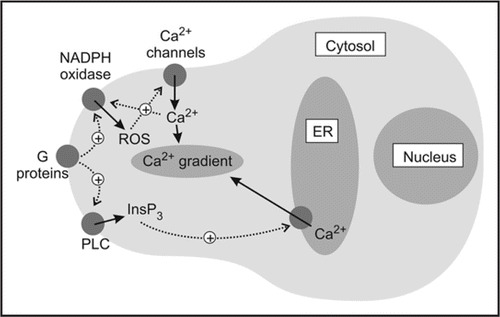
Our results strongly suggested that the Ca2+ gradient was indeed interdependent on ROS generation by NADPH oxidases and supported both our earlier work in root hairs11 and the work done by Nicholas Smirnoff's and Victor Žárský's groups on pollen tubes.Citation12 Taken together, our data are consistent with a model in which ROS stimulate generation of a tip-high Ca2+ gradient which is amplified by positive feedback between Ca2+ and ROS production and then maintained by InsP3 activity ().
Several questions remain. First, our paper looked briefly at a possible role for PLC and InsP3 in supporting the intracellular Ca2+ gradient; our conclusion that PLC acted through its product, InsP3, in F. serratus zygotes does not agree with work done in flowering plant pollen tubes, in which PLC is believed to act through its substrate, PIP2.Citation16,Citation17 Whether this is a species- or cell-specific difference remains to be determined.
Second, our paper only looked at the interdependence of Ca2+ and ROS in zygotes during the third and final stage of polarity establishment—the germination of the rhizoid. There is some evidence that redox processes are also involved in axis formationCitation18 and it would be interesting to know whether the ROS and Ca2+ signaling networks are interdependent during axis formation and fixation.
Third, and finally, the next challenge is to integrate these findings on small molecule gradients with the better characterized work on protein gradients in order to understand how different signaling and communication pathways set up a regulatory network.Citation19 With this aim in mind, the traditional model organisms in which polarity has been studied may need to be updated. What is now needed is a model with easily visualized zygotes and a tractable genome which will allow a combination of genetic and physiological approaches. Surprisingly, the best placed candidates may be the moss, Physcomitrella patensCitation20 and the brown alga, Ectocarpus siliculosusCitation21,Citation22 and we look forward to seeing what their futures hold.
Addendum to:
References
- Huynh JR, St Johnston D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr Biol 2004; 14:438 - 449
- Heasman J. Maternal determinants of embryonic cell fate. Semin Cell Dev Biol 2006; 17:93 - 98
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 2003; 426:147 - 153
- Weijers D, Jurgens G. Auxin and embryo axis formation: the ends in sight?. Curr Opin Plant Biol 2005; 8:32 - 37
- Brownlee C, Bouget FY. Polarity determination in Fucus: from zygote to multicellular embryo. Semin Cell Dev Biol 1998; 9:179 - 185
- Hable WE, Kropf DL. Sperm entry induces polarity in fucoid zygotes. Development 2000; 127:493 - 501
- Hurd AM. Effect of unilateral monochromatic light and group orientation on the polarity of germinating Fucus spores. Bot Gaz 1920; 70:25 - 50
- Corellou F, Potin P, Brownlee C, Kloareg B, Bouget FY. Inhibition of the establishment of zygotic polarity by protein tyrosine kinase inhibitors leads to an alteration of embryo pattern in Fucus. Dev Biol 2000; 219:165 - 182
- Berger F, Brownlee C. Ratio confocal imaging of free cytoplasmic calcium gradients in polarising and polarised Fucus zygotes. Zygote 1993; 1:9 - 15
- Pu R, Robinson KR. Cytoplasmic calcium gradients and calmodulin in the early development of the fucoid alga Pelvetia compressa. J Cell Sci 1998; 111:3197 - 3207
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003; 422:442 - 446
- Potocky M, Jones MA, Bezvoda R, Smirnoff N, Zarsky V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 2007; 174:742 - 751
- Mori IC, Schroeder JI. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 2004; 135:702 - 708
- Bothwell JH, Ng CK. The evolution of Ca2+ signalling in photosynthetic eukaryotes. New Phytol 2005; 166:21 - 38
- Rossetti S, Bonatti PM. In situ histochemical monitoring of ozone- and TMV-induced reactive oxygen species in tobacco leaves. Plant Physiol Biochem 2001; 39:433 - 442
- Dowd PE, Coursol S, Skirpan AL, Kao TH, Gilroy S. Petunia phospholipase C1 is involved in pollen tube growth. Plant Cell 2006; 18:1438 - 1453
- Helling D, Possart A, Cottier S, Klahre U, Kost B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 2006; 18:3519 - 3534
- Berger F, Brownlee C. Photopolarization of the Fucus sp zygote by blue-light involves a plasma-membranerRedox chain. Plant Physiol 1994; 105:519 - 527
- Brenner ED, Stahlberg R, Mancuso S, Vivanco J, Baluska F, Van Volkenburgh E. Plant neurobiology: an integrated view of plant signaling. Trends Plant Sci 2006; 11:413 - 419
- Cove D. The moss Physcomitrella patens. Ann Rev Genet 2005; 39:339 - 358
- Charrier B, Coelho SM, Le Bail A, Tonon T, Michel G, Potin P, Kloareg B, Boyen C, Peters AF, Cock JM. Development and physiology of the brown alga Ectocarpus siliculosus: two centuries of research. New Phytol 2008; 177:319 - 332
- Peters AF, Marie D, Scornet D, Kloareg B, Cock JM. Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. J Phycol 2004; 40:1079 - 1088