 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Phyllotaxis, the arrangement of a plant's phylla (flowers, bracts, stickers) near its shoot apical meristem (SAM), has intrigued natural scientists for centuries. Even today, the reasons for the observed patterns and their special properties, the physical and chemical mechanisms which give rise to strikingly similar configurations in a wide variety of plants, the almost-constant golden divergence angle, the almost constant plastichrone ratio, the choices of parastichy numbers and the prevalence of Fibonacci sequences to which their numbers belong, are at best only partially understood. Our goals in this Addendum are: i) To give a brief overview of current thinking on possible mechanisms for primordia (the bumps on the plant surface which eventually mature into fully developed structures such as leaves or florets) formation and give a descriptive narrative of the mathematical models which encode various hypotheses. ii) To emphasize the point that patterns, whether they be phyllotactic configurations on plant surfaces or convection cells on the sun's surface, are macroscopic objects whose behaviors are determined more by symmetries of the proposed model and less by microscopic details. Because of this, the identification of observations with the predications of a particular model can only be made with confidence when the match coincides over a range of circumstances and parameters. iii) To discuss some of the key results of the proposed models and, in particular, introduce the prediction of a new and, in principle, measurable invariant in plant phyllotaxis. iv) To introduce a new model of primordia formation which is more in keeping with the pictures and paradigms of Hofmeister,1 Snow & Snow,2 and Douady and Couder3,4 which see primordia as forming in a fairly narrow annular zone surrounding the plant's SAM separating a region of undifferentiated cells from a fully developed patterned state. v) To consider the challenge of phyllotaxis in the broader context of pattern formation in biological tissue which responds to both mechanical and biochemical processes.
Addendum to: Newell AC, Shipman P, Sun Z. Phyllotaxis: Cooperation and competition between biomechnical and biochemical processes. J Theor Biol 2008; 251:421-39.
Experiments involving the applications of the hormone auxin and its inhibitorsCitation5–Citation8 have established that auxin plays an important role in primordia formation. But only in the last few years has it been understood how a uniform concentration of auxin could give rise to an inhomogeneous quasi-periodic pattern of enhanced and depleted auxin zones. The key idea for this instability, vividly seen visually in the beautiful experiments of Kuhlemeier and his groupCitation5–Citation8 and Meyerowitz et al.,Citation9 on Arabidopsis, is that the PIN1 proteins in each cell on the plant's surface can be regulated by the relative distributions of auxin in neighboring cells so that these proteins gravitate to and polarize in the cell walls. The net effect is to drive auxin in the direction of its concentration gradient. This reverse diffusion gives rise to instability of a spatially uniform auxin concentration state and a pattern whose wavelength, approximately 12 to 15 cell diameters, is determined by a balance of auxin loss to the plant body and PIN1-mediated transport.
Elaborate mathematical models of Jönsson et al.,Citation9 simulate and indeed capture much of the overall dynamics in detail and show the beginning of a quasi-periodic array of centers of auxin enhancement and depletion. But many important questions remain open. In the experiments, the plant surface clearly undergoes significant deformation as the regions of primordia formation begin to bulge out into fully developed structures. No such surface deformations are treated in the model of Jönnson et al.,Citation9 Further, it has been shown in many plants, for example, sunflowers, that growing tissue creates compressive stresses in the plant's tunica.Citation10 Indeed, Green and othersCitation11–Citation17 have argued that the compressive stresses are responsible for producing a buckling of the tunica. In particular, they suggested that the circumferential compressive stress due to the growth of the annular primordia forming region in the neighborhood of the SAM was the main trigger for primordia growth. Their argument was that the inhomogeneous stress distribution associated with the buckled tunica surface stimulates an inhomogeneous production of hormones which promotes the growth of primordia in the regions of enhanced hormone concentration. A somewhat different role for stresses, whereby a variation in the tension field in the tunica (caused by variation in auxin distribution) leads to primordium bump formation, is discussed by Fleming.Citation18
In order to address the question of the cooperation and competition of mechanical and biochemical processes, we developed a mathematical model which includes both.Citation19 Our model takes advantages of the observations in Arabidopsis that the pattern wavelength is large with respect to the cell diameter.Citation9 We can therefore legitimately use continuous (rather than discrete) field variables to represent both the local auxin concentrations and surface deformation. Fluctuations in auxin concentration influence the mechanical forces in the tunica by creating uneven growth and are manifested by an additional strain contribution in the stress-strain relationships. On the other hand, inhomogeneities in the stress distribution are assumed to lead to changes in auxin concentration. The exact way in which stresses influence biological tissue growth (weight-bearing bones and fruit stems become stronger) is still an open challenge to biologists. We simply assume that the auxin-produced growth is proportional, in a first approximation, to how much average tensile stress the local elemental volume (which will contain many cells) feels. This is best measured by the trace of the stress tensor at that location.
We analyze the model by imagining that the domain of interest, an annular region in the neighborhood of the SAM, is sufficiently large to accommodate many pattern wavelengths. Then we represent the surface deformation and auxin fluctuation fields by a combination of quasi-periodic Fourier modes whose angular wave numbers m are integers. The amplitudes of each of the modes are called order parameters and play the same role as similarly named variables do in the description of phase transitions. A great simplification of the model (and the universality of pattern characteristic behaviors) is seen near the onset of primordium formation where one or both of the two most important parameters, encoding how far the system is from equilibrium, are near their threshold values. Namely, we assume either or both of the PIN1-transport coefficient leading to reverse diffusion and the growth-induced circumferential stress are near critical values. In that case, the order parameter amplitudes satisfy simple, nonlinear algebraic equations which are multinomial Taylor expansions in the amplitudes of the participating modes and the amounts by which the important parameters are super- or subcritical. All the microscopic details, the radius of the active annular region, the circumferential stress, PIN1 transport, regular auxin diffusion and loss of auxin to the bulk, the elastic properties of the tunica, are contained in the coefficients of the order parameter equations (equations (3.3) in ref. Citation19).
This is a very important point we wish to stress. Many different pictures and hypotheses as to the origin of phyllotaxis will yield very similar sets of order-parameter equations. The shape of the equations, namely which terms appear, or do not appear, only depends on certain symmetries the models have in common. The devil is in the details. To say that one or other particular model is the correct one requires one to show that the observed features, the phyllotactic pattern in many different plants, the associated surface deformations and surface tilings, the changes in the pattern as the plant grows, are matched by theory. Moreover, the job of theory is not only to identify the correct model from a mechanistic point of view but to find the simplest nontrivial model which captures most of the observed behaviors. Describing patterns in which changes occur over cms in terms of thousands of basic elements which have length scales of microns does not usually lead to enhanced understanding of the patterns themselves.
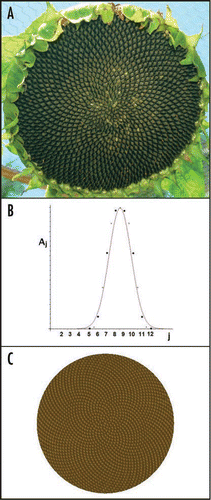
While our model is perhaps too simple, it does produce several significant results, two of which we discuss here. First, it explains how in some plants, for example, cacti, the surface deformations, which may be ridge-dominated, can be different from the phyllotactic configuration in which the pattern consists of flowers and stickers lying on families of spirals. What our analysis shows is that, if the circumferential stress in the formative region is subcritical (the region may even be in tension) but the PIN1 transport is supercritical, the surface deformation will be slaved to the phyllotactic configuration. Namely, the surface deformation will have its maxima and minima at maxima and minima of auxin concentration. But as the circumferential stress nears its critical value and the natural instability wavelength of the two instabilities match, there is cooperation between the processes and the surface deformation and auxin concentration field patterns can be quite different. In our recently published paper,Citation19 we document several examples (reviewed in refs. Citation8–Citation19). Second, we have discovered a new plant invariant which highlights the self-similar nature of the plant patterns one sees, for example, in the diamond and offset-diamond tilings of a sunflower head.Citation20,Citation21 The invariant follows from a set of symmetries in the order parameter equations in which the set of equations remains invariant under transformations which simulate moving radially outward in the sunflower head. If we imagine that the set of participating modes is enumerated by the circumferential wavenumbers m belonging to the regular Fibonacci sequence (we and others have explained elsewhere why this sequence is preferred), then at different radii on the sunflower head, different sets of modes m will play dominant roles. The reason for this is that the pattern wavelength is an intrinsic constant only depending on plant parameters. Therefore, more and more of these sinusoidal oscillations fit around the annulus the further out one goes. It turns out that, at any given radius, the pattern is shaped by four modes, two dominant, two less dominant, (all other modes at this radius have very small amplitudes) whose circumferential wave numbers m1, m2, m3 = m1 + m2, m4 = m1 + 2m2 are sequential members of the Fibonacci sequence. If we draw the graph of the amplitudes as function of m, the circumferential wavenumbers (as in ) , this graph will have the same shape at all radii although the participating wavenumbers move down the Fibonacci sequence as we move towards the center of the sunflower head. In , we compare an actual sunflower head () with our theoretical replica ().
To this point, our model has assumed that the phyllotactic configurations and the surface deformation all form in a large annulus more or less simultaneously. This is not what happens. In real plant growth, the center of the meristem consists of a less-stiff region of undifferentiated cells where new cells are created and which become increasingly differentiated near a certain radius r = R1(t) as they take on the stiff nature of the plant's tunica. A simple pattern, usually of decussate phyllotaxis, forms in the annular region R1(t), R2(t) where r = R2(t) is the outer region of the meristem. Both R1(t), R2(t) grow with time, the former more slowly because of the transition from undifferentiated to differentiated cells. As new tunica surface is created at r = R1(t), the new primordia are initiated there, namely in a very narrow annulus (about one wavelength λ in radial width) between the region r < R1(t) of undifferentiated cells and the region R2(t) > r > R1(t) + λ of a fully developed pattern.
As the radius at which new primordia are formed increases, the configuration will evolve, from a decussate configuration on the outside to a spiral form of phyllotaxis with higher and higher Fibonacci numbers on the inside. This enumeration is reversed in some plants; e.g., the sunflower (reviewed in ref. Citation22). In order to model this picture, we now treat these regions separately and allow for solutions of our equations which capture the synchronization of moving fronts. One front connects the region of undifferentiated cells with the undeformed tunica surface. The other connects the latter, which is unstable to the patterned state, with the patterned state. Our simulations to date have produced patterns very similar to what is observed. They also bring us more in line with the paradigms and pictures of Hofmeister,Citation1 Snow & Snow,Citation2 and Douady and Couder.Citation3,Citation4
Finally we want to point out that while phyllotaxis is perhaps the granddaddy of pattern-forming systems in biological tissues, there are many other situations where the pattern is produced by a symbiosis between growth-induced mechanical forces and biochemical processes.Citation23,Citation24 In particular, we note that the epidermal ridges on our fingertips are formed by a combination of mechanical forces in the epidermal skin layer initiated by the collapse of volar pads in the foetus stage of life and the nonuniform distribution of Merkel cells in the basal layer which appears to play a role in the formation and utility of the patterns of nerve filaments which carry information from our fingers to our central nervous system. These nerve endings end in concentrations of Merkel cells which lie along the epidermal ridges in hexagonal lattices. Whereas the reason for the mechanical instability is understood (buckling of the epidermal layerCitation25), we do not yet understand the analogy of PIN1 reverse diffusion, namely what leads to the biochemical instability (chemotaxy?—haptotaxy?—A mechanism akin to PIN1?) to a nonuniform pattern of Merkel cells.
We are at the beginning of a very exciting state in biology where we might at last appreciate the symbiosis between mechanical and biochemical processes in producing self-organizing behavior in biological tissues on scales far larger than those of genes and cells.
Figures and Tables
Figure 1 (A) A sunflower seed head. The seeds trace out families of spirals in the Fibonacci sequence. Photo courtesty of John Palmer. (B) A theoretical invariant curve A(r,j) (in red) which gives the amplitude Aj of the Fourier mode of angular wavenumber mj at radius r from the apex center. The curve is plotted as a function of j for fixed r. As r increases, the curve moves to the right but does not change shape. In dots are a calculation of the invariant curve from the order parameter equations, as described recently.Citation20,Citation21 (C) The graph of the Fourier approximation,
of the plant surface where the mj lie in the Fibonacci sequence. The r-dependent radial wavenumbers lj and a derivation of the curve A(r,j) are given in reference Citation21. (B and C) are reproduced with permission from reference Citation20.
Acknowledgements
This work is supported by NSF Grants DMS 0501243 and DMS 0503196.
Addendum to:
References
- Hofmeister W. Allgemeine Morphologie der Gewachse, Handbuch der Physiologischen Botanik 1868; Leipzig Engelmann
- Snow M, Snow R. Minimum areas and leaf determination. Proc R Soc B 1952; 139:545 - 566
- Douady S, Couder Y. Phyllotaxis as a dynamical self-organizing process I. The spiral modes resulting from time-periodic iterations. J Theor Biol 1996; 178:255 - 274
- Douady S, Couder Y. Phyllotaxis as a dynamical self-organizing process II. The spontaneous formation of a periodicity and the coexistance of spiral and whorled patterns. J Theor Biol 1996; 178:295 - 312
- Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C. Induction of leaf primordia by the cell wall protein expansin. Science 1997; 276:1415 - 1418
- Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of lateral organs. Plant Cell 2000; 12:501 - 518
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature 2003; 426:255 - 260
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. A plausible model of phyllotaxis. Proc Natl Acad Sci USA 2006; 103:1301 - 1306
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjölsness E. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA 2006; 103:1633 - 1638
- Dumais J, Steele CR. New evidence for the role of mechanical forces in the shoot apical meristem. J Plant Growth Regul 2000; 19:7 - 18
- Green PB. Expression of pattern in plants: Combining molecular and calculus-based paradigms. Am J Bot 1999; 86:1059 - 1076
- Green PB, Cummins WR. Growth rate and turgor pressure: Auxin effect studied with an automated apparatus for single coleoptiles. Plant Physiol 1974; 54:863 - 869
- Green PB, Steele CS, Rennich SC. Jean RV, Barabé D. How plants produce patterns. A review and a proposal that undulating field behavior is the mechanism. Symmetry in Plants 1998; Singapore World Scientific 359 - 392
- Hernández LH, Green PB. Transductions for the expression of structural pattern: Analysis in sunflower. Plant Cell 1993; 5:1725 - 1738
- Shipman PD, Newell AC. Phyllotactic patterns on plants. Phys Rev Lett 2004; 92:168102
- Shipman PD, Newell AC. Polygonal planforms and phyllotaxis on plants. J Theor Biol 2004; 236:154 - 197
- Steele CR. Shell stability related to pattern formation in plants. J Appl Mech 2000; 67:237 - 247
- Fleming AJ. Leaf initiation: the integration of growth and cell division. Plant Mol Biol 2006; 60:905 - 914
- Newell AC, Shipman PD, Sun Z. Phyllotaxis: Cooperation and competition between biomechanical and biochemical processes. J Theor Biol 2008; 251:421 - 439
- Newell AC, Shipman PD. A new invariant in plant phyllotaxis. Anal Appl 2008; accepted for publication
- Shipman PD. Discrete and continuous invariance in phyllotactic tilings (Unpublished Data)
- Dosio GAA, Tardieu F, Turc O. How does the meristem of sunflower capitulum cope with tissue expansion and floret initiation? A quantitative analysis. New Phytol 2006; 170:711 - 722
- Braam J. In touch: plant responses to mechanical stimuli. New Phytol 2005; 165:373 - 389
- Brouzés E, Farge E. Interplay of mechanical deformation and patterned gene expression in developing embryos. Curr Opin Genet Dev 2004; 14:367 - 374
- Kücken M, Newell AC. Fingerprint formation. J Theor Biol 2005; 235:71 - 83