Abstract
Our paper describes how the genetic pathways which regulate vernalization and long-day flowering responses are integrated to promote spring flowering in cereals. This process is mediated by the VERNALIZATION1 (VRN1) and VRN2 genes. VRN2 encodes a CONSTANS-like protein that represses FLOWERING LOCUS T (FT1) to block the long-day flowering response until plants are vernalized. When plants are vernalized VRN1, a FRUITFUL-like MADS box transcription factor, is induced. This down-regulates VRN2, allowing long-day induction of FT1 to occur post-vernalization. A comparison of the pathways regulating seasonal induction of flowering in cereals with those of Arabidopsis shows the vernalization response pathway has evolved convergently to regulate the activity of a conserved daylength response pathway in these divergent plant lineages.
Addendum to: Hemming M.N, Peacock W.J, Dennis E.S, Trevaskis B. Low temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol 2008; 147:355-66.
The Seasonal Flowering Responses of Arabidopsis
Flowering of Arabidopsis is accelerated by long-days, a process mediated by FLOWERING LOCUS T (FT). FT encodes a phospatidylethanolamine binding protein produced in leaves in long days, then transported to the shoot apex to promote floral development.Citation1–Citation3 Long-day induction of FT is controlled by CONSTANS (CO).Citation4 The level of CO mRNA oscillates diurnally reaching a peak in late afternoon and CO protein is stabilised by light.Citation4,Citation5 In long-days the diurnal peak in CO mRNA levels overlaps with daylight so CO activates FT expression.Citation4,Citation5
In many Arabidopsis ecotypes, the long-day flowering response is suppressed until plants are exposed to low temperatures for prolonged periods over winter (vernalization). In these ecotypes a MADS box transcription factor encoded by FLOWERING LOCUS C (FLC) represses FT to block the long-day response until plants are vernalized.Citation6,Citation7 Expression of FLC decreases when plants are vernalized during winter, allowing subsequent long-day induction of FT to promote flowering in spring.Citation6,Citation7 Vernalization-induced repression of FLC is mediated by histone modification complexes that change the state of chromatin at the FLC locus resulting in reduced transcription of FLC.Citation8,Citation9 Modifications to FLC chromatin are mitotically stable, so a cellular memory of vernalization is maintained until the next generation.Citation10 Such a mechanism makes sense in a seasonal context; repression of FLC must be maintained after winter as temperatures and daylength increase in spring.
The Seasonal Flowering Responses of Temperate Cereals
Flowering of temperate cereals, such as wheat and barley, can be accelerated by long-days. This is mediated by orthologues of the Arabidopsis CO and FT genes.Citation11 Vernalization also accelerates flowering of many wheat and barley varieties. To understand how long-day and vernalization flowering responses are integrated we examined expression of an orthologue of the Arabidopsis FT gene (HvFT1) in a vernalization-responsive barley.Citation12 We found that prior to vernalization expression of HvFT1 is not induced by long days. Moreover, HvFT1 is not induced by low temperatures during vernalization treatment. Instead, HvFT1 expression increases only when plants that have been vernalized are subsequently exposed to long-days. Thus, like Arabidopsis, cereals remember vernalization and this allows long days to promote flowering.
In barley, the VERNALIZATION1 (HvVRN1) and HvVRN2 genes determine whether a variety requires vernalization.Citation13 Another gene, PHOTOPERIOD1 (PPD1) mediates long-day induction of HvFT1 and determines whether plants show a strong long-day flowering response.Citation11 We examined how interactions between these three genes influence expression of HvFT1.Citation12 Our data show that HvVRN2 downregulates HvFT1 to suppress the long-day flowering response until plants are vernalized. If HvVRN2 activity is abolished, through deletion of the HvVRN2 gene, HvFT1 is expressed in long days without prior vernalization. This bypasses the normal requirement for vernalization and causes early flowering; an effect that is strongest in lines with the active PPD1 gene. Conversely, overexpression of HvVRN2 downregulates HvFT1 and delays flowering in transgenic barley.
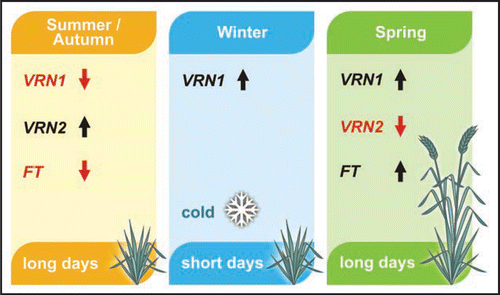
HvVRN2 is repressed in vernalized barley plants,Citation14–Citation16 and this can explain why long-days are able to induce HvFT1 expression postvernalization. It is unlikely, however, that low-temperatures directly regulate expression of HvVRN2. HvVRN2 is regulated primarily by daylength and is not expressed in short-day conditions that are typical of winter.Citation16 Instead, the low-temperature response is likely to be mediated by HvVRN1.Citation16,Citation17 In vernalization responsive varieties, HvVRN1 is induced by vernalization, irrespective of daylength, and HvVRN1 expression levels remain high post-vernalization.Citation15 Moreover, genetic data suggest that activation of HvVRN1 is the basis of vernalization-induced flowering. There are alleles of HvVRN1 that are expressed at high levels without prior cold treatment,Citation17,Citation18 and the effect of these alleles mimics vernalization; HvVRN2 is repressed, HvFT1 is expressed in long days without prior cold treatment and plants flower early without vernalization.Citation12 Thus, in vernalization-responsive barleys, low-temperature induced HvVRN1 expression is likely to repress HvVRN2 post-vernalization and allow increased expression of HvFT1 in long days ().
Common Themes of the Vernalization Response in Arabidopsis and the Temperate Cereals
In both Arabidopsis and cereals a key feature of seasonal flowering control is suppression of long-day induction of FT until plants over-winter. In Arabidopsis a single gene, FLC, both represses FT and responds to low-temperatures during winter to allow subsequent long-day induction of FT. In cereals two separate genes fulfil these roles; VRN2, a long-day specific floral repressor, downregulates FT1 before winter, and another gene, VRN1, responds to low-temperatures during winter. Although different genes mediate the vernalization response in these divergent plant lineages, it seems that this response has evolved convergently through regulation of a conserved daylength response pathway.
There might be other mechanistic similarities between the vernalization responses of Arabidopsis and the cereals. Regions within the first intron of the FLC gene are targeted by the histone modification complexes that mediate vernalization-induced repression of FLC expression and are essential for maintained repression of FLC.Citation8,Citation9,Citation19 In cereals, deletions in the first intron of VRN1 are associated with derepression of VRN1 expression,Citation20 suggesting that similar molecular mechanisms might target the VRN1 locus prior to winter to prevent precocious expression of VRN1 and maintain the requirement for vernalization. Thus, epigenetic regulation may be a feature of the vernalization response in both Arabidopsis and cereals, but with opposite roles.
Conclusion and Perspective
Flowering time is an important trait in cereals. Understanding how long-day and vernalization responses are integrated will be useful for the development of cereal breeding strategies and offers insights into the evolution of the vernalization response. It will now be possible to examine whether VRN1 directly represses VRN2 and if VRN2 directly represses FT1. Analysis of histone modifications at the VRN1 locus could also reveal potential epigenetic regulation of VRN1 in vernalization responsive cereals.
Figures and Tables
Figure 1 The molecular basis of spring flowering in temperate cereals. Prior to winter the vernalization and long-day flowering response pathways are inactive because VRN1 is not expressed and VRN2 represses FT1 in long days. Hence flowering is delayed. Prolonged exposure to cold over winter induces expression of VRN1, while FT1 remains inactive in the short days of winter. Following winter, expression of VRN1 remains high. This promotes inflorescence initiation and also downregulates VRN2 to allow long-day induction of FT1 as days lengthen. This promotes rapid flowering in spring.
Addendum to:
References
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science 1999; 286:1962 - 1965
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 1999; 286:1960 - 1962
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007; 316:1030 - 1033
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 2001; 410:1116 - 1120
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004; 303:1003 - 1006
- Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999; 11:949 - 956
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JE, Peacock WJ, Dennis ES. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 1999; 11:445 - 458
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 2004; 427:164 - 167
- Sung S, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 2004; 427:159 - 164
- Sheldon CC, Hills MJ, Lister C, Dean C, Dennis ES, Peacock WJ. Resetting of FLOWERING LOCUS C expression after epigenetic repression by vernalization. Proc Natl Acad Sci USA 2008; 105:2214 - 2219
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 2005; 310:1031 - 1034
- Hemming MN, Peacock WJ, Dennis ES, Trevaskis B. Low temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol 2008; 147:355 - 366
- Takahashi R, Yasuda S. Nilan RA. Genetics of earliness and growth habit in barley. Barley genetics II. (Proceedings of the second international barley genetics symposium) 1971; Pullman Washington State University Press 388 - 408
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. The wheat VRN2 gene is a flowering repressor downregulated by vernalization. Science 2004; 303:1640 - 1644
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 2006; 103:19581 - 19586
- Trevaskis B, Hemming MN, Peacock WJ, Dennis ES. HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 2006; 140:1397 - 1405
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci 2007; 12:353 - 357
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES. MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 2003; 100:13099 - 13104
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 2003; 100:6263 - 6268
- Sheldon CC, et al. Quantitative effects of vernalization on FLC and SOC1 expression. Plant J 2006; 45:871 - 883
- Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genomics 2005; 273:54 - 65