Abstract
Protein kinase CK2 is a pleitropic Ser/Thr kinase present in all eukaryotes. In order to study the effects of CK2 depletion on plant development, we have recently generated Arabidopsis transgenic plants expressing a CK2α-inactive mutant under the control of an inducible promoter. Our results showed that continuous expression of the transgene had a dominant negative effect and was lethal for Arabidopsis plants. Overexpression of the CK2α-inactive subunit provoked cell cycle arrest, by perturbation of both G1/S and G2 cell-cycle phases. The effects on cell division were particularly strong in root meristems, causing inhibition of lateral root formation even when the mutant protein was transiently induced. Processes that rely on cell expansion, such as hypocotyl elongation in dark-grown seedlings, were also strongly affected. We propose that CK2 regulates auxin-signaling pathways.
Addendum to: Moreno-Romero J, Carme Espunya M, Platara M, Ariño J, Carmen Martínez M. A role for protein kinase CK2 in plant development: evidence obtained using a dominant-negative mutant. Plant J. 2008; In press.
Protein phosphorylation-dephosphorylation is one of the most important mechanisms of cell signaling, which allows rapid responses to a particular stimulus because no new protein synthesis is needed. Protein kinase CK2 is among the earliest protein kinases ever discovered.Citation1 It is ubiquitously present in eukaryotic cells and highly conserved among species.Citation2 In spite of hundred of studies performed in different species, the particular function of CK2 is still not known, nor how its enzymatic activity is regulated within the cell. A fundamental role of CK2 in processes such as gene expression, cell cycle control and DNA repair has been recently highlighted,Citation3 which agrees with its predominant nuclear localization. In addition, CK2 phosphorylates transcription factors involved in photomorphogenesis,Citation4 and CK2 activity is required in abcisic acid and salicylic acid signaling pathways.Citation5,Citation6 The pleitropic nature of CK2 has been an obstacle to understand its physiological function. One possibility is that some of the substrates of CK2 are common regulators of different signaling pathways and thus act as integrators of transcriptional networks. The identification of HY5 as a physiological substrate of CK2Citation4 supports this idea. HY5 is a transcription factor that appears to integrate light and hormone signaling pathways.Citation7,Citation8 Phosphorylation by CK2 influences HY5 protein stability by modulating its interaction with the COP9 signalosome.
CK2 exists in different isoforms in all eukaryotes, and this multiplicity is especially high in plants.Citation9,Citation10 To study the impact of CK2 activity depletion upon Arabidopsis development we have generated a loss-of-function mutant. Due to the absolute requirement of CK2 activity for life,Citation11 and the partially redundant functions of the different CK2 isoforms,Citation12 we decided to use a dominant negative mutant approach. A CK2α kinase-inactive subunit was constructed (CKA3mut) and cloned downstream of a glucocorticoid-inducible promoter. Stably transformed Arabidopsis plants were obtained with this construct. As expected, long-time inductions of the transgene were lethal for the plants, causing growth and development arrests, and ultimately resulting in plant death. However, short-time inductions were not lethal, and revealed interesting phenotypical changes that were the consequence of blocking development processes during a temporal window.Citation13 Here we want to highlight two interesting characteristics of the mutant, the apparent de-etiolated phenotype of dark-grown seedlings, and the inhibition of lateral root formation.
Dark-grown mutant plants exhibited an apparent de-etiolated phenotype, the main characteristics of which were short hypocotyls and opening and expansion of cotyledons (). Scanning electron microscopy revealed a strict correlation between the size of hypocotyls and cotyledons and that of the corresponding epithelial cells. Other typical features of de-etiolated mutants were here absent, such as a constitutive activation of light-induced genes (RBCS, CAB or CHS) or chloroplast development. Moreover, presence of light did not lead to chloroplast development nor to the induction of RBCS or CAB transcription. However, levels of CHS transcripts were normal in the light-grown mutant, indicating that light perception and signaling were not impaired. These results strongly suggest a defect in cell expansion/elongation in the mutant, and also uncoupling of light signaling with typical developmental responses, such as chloroplast development or onset of the de-etiolation program.
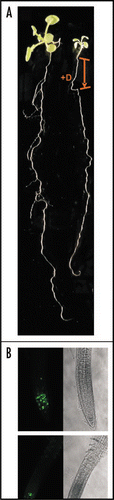
Strong inhibition of lateral root formation was the most significant phenotype observed by short-time inductions of the transgene (). Interestingly, appearance of lateral roots was for a long time impaired, even after dexamethasone removal, both in the portion of the root that was exposed to the inducer and in the new segment that appeared afterwards. Only 2–3 weeks after dexamethasone removal, some root primordia started to grow (results not shown). Using the mitotic reporter CYCB1;1::GFP, we demonstrated a cell cycle arrest in the root apical meristem after CKA3mut induction. Moreover, expression of CKA3mut in BY2 cells revealed perturbation of G1/S and G2 phases of the cell cycle, which is coincident with the position of CK2 activity peaks during BY2 cell cycle previously reported.Citation14
Auxin plays a prominent role in lateral root formation. Pericycle cells at the site of future primordia initiation start accumulating auxin,Citation15 then cell division is activated and subsequently auxin transport is redirected to the growing primordia. Thus, dynamic auxin gradients, originated by polar auxin transport that lead to differential auxin distribution along the root, are essential for root morphogenesis, promoting either cell division or cell elongation in the different zones of the roots.Citation16,Citation17 Auxin polar transport involves specific efflux carriers (PIN proteins).Citation17 By blocking the cell cycle after induction of CKA3mut, we are probably affecting to the initiation of transversal divisions in pericycle cells, necessary for root primordia formation. We propose that the CKA3mut effect on cell division might be exerted through affecting auxin gradients, which could explain the long-lasting effects on the lateral root phenotype and the cell expansion defects observed in seedlings.
Figures and Tables
Figure 1 Effects of CK2 depletion on cellular expansion. (A) Hypocotyl lengths and cotyledon areas of wild type (WT) and mutant plants (mut), germinated and grown for four days in darkness, with or without dexamethasone (±D). (B) Phenotypic characteristics of dexamethasone-treated mutant plants, grown either under long-day conditions (LD) or in darkness. Four-day-old plantlets are shown. Bar: 1 mm. (C) Scanning electron microscopy images of hypocotyl and cotyledon surfaces of mutant plants, grown either under long-day (LD) conditions or in darkness, and with or without dexamethasone (±D). Some cells have been marked with contour lines to facilitate their view.
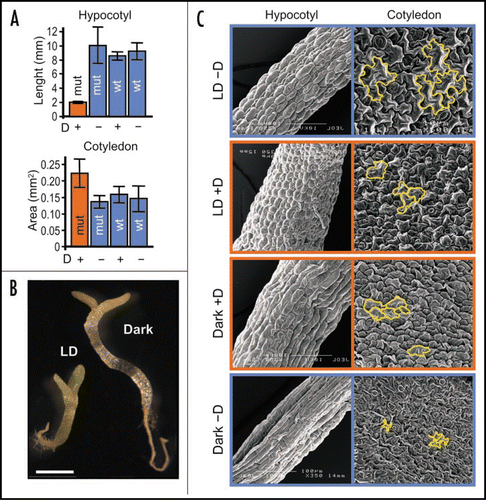
Figure 2 Effects of CK2 depletion on lateral roots formation. (A) Fifteen-day-old mutant plants, either untreated (left) or treated (right) with dexamethasone (+D). Dexamethasone-treatment was performed for 48 hours in five-day-old seedlings. The arrow indicates the portion of the root that was directly exposed to dexamethasone, whereas the segment below appeared after dexametasone removal. (B) CycB1;1::GFP expression in apical root meristems of seven-day-old mutant plants, either untreated (above) or treated (below) with dexamethasone.
Addendum to:
References
- Burnett G, Kennedy EP. The enzymatic phosphorylation of proteins. J Biol Chem 1954; 211:969 - 980
- Litchfield DW. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem J 2003; 369:1 - 15
- Pyerin W, Ackermann K. The genes encoding human protein kinase CK2 and their functional links. Prog Nucleic Acid Res Mol Biol 2003; 74:239 - 273
- Hardtke CS, Gohda K, Osterlund MT, Oyama T, Okada K, Deng XW. HY5 stability and activity in arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J 2000; 19:4997 - 5006
- Riera M, Figueras M, Lopez C, Goday A, Pages M. Protein kinase CK2 modulates developmental functions of the abscisic acid responsive protein Rab17 from maize. Proc Natl Acad Sci USA 2004; 101:9879 - 9884
- Hidalgo P, Garreton V, Berrios CG, Ojeda H, Jordana X, Holuigue L. A nuclear casein kinase 2 activity is involved in early events of transcriptional activation induced by salicylic acid in tobacco. Plant Physiol 2001; 125:396 - 405
- Cluis CP, Mouchel CF, Hardtke CS. The arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 2004; 38:332 - 347
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 2007; 19:731 - 749
- Salinas P, Fuentes D, Vidal E, Jordana X, Echeverria M, Holuigue L. An extensive survey of CK2 alpha and beta subunits in arabidopsis: Multiple isoforms exhibit differential subcellular localization. Plant Cell Physiol 2006; 47:1295 - 1308
- Espunya MC, Lopez-Giraldez T, Hernan I, Carballo M, Martinez MC. Differential expression of genes encoding protein kinase CK2 subunits in the plant cell cycle. J Exp Bot 2005; 56:3183 - 3192
- Padmanabha R, Chen-Wu JL, Hanna DE, Glover CV. Isolation, sequencing and disruption of the yeast CKA2 gene: Casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol 1990; 10:4089 - 4099
- Barz T, Ackermann K, Dubois G, Eils R, Pyerin W. Genome-wide expression screens indicate a global role for protein kinase CK2 in chromatin remodeling. J Cell Sci 2003; 116:1563 - 1577
- Moreno-Romero J, Carme Espunya M, Platara M, Ariño J, Carmen Martínez M. A role for protein kinase CK2 in plant development: Evidence obtained using a dominant negative mutant. Plant J 2008; 55:118 - 130
- Espunya MC, Combettes B, Dot J, Chaubet-Gigot N, Martinez MC. Cell cycle modulation of CK2 activity in tobacco BY-2 cells. Plant J 1999; 19:655 - 666
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003; 115:591 - 602
- Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 2007; 449:1008 - 1013
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005; 433:39 - 44