Abstract
Trichoderma spp. are effective biocontrol agents for numerous foliar and root phytopathogens, and some are also known for their abilities to enhance systemic resistance to plant diseases as well as overall plant growth. Root colonization with T. harzianum strain T22 induces large changes in the proteome of shoots of maize seedlings (Zea mays) even though T22 is present only on roots; changes also were recorded in the roots. In shoots, we identified 91 of 114 upregulated and 30 of 50 downregulated proteins. In roots, 20 upregulated and 11 downregulated proteins were found and 17 and eight, respectively, were identified. Classification of proteins differentially expressed in both shoots and roots revealed that the largest number of upregulated proteins was involved in carbohydrate metabolism; in shoots, some upregulated proteins were involved in photosynthesis. Increases in these protein classifications suggest enhanced respiratory and photosynthetic rates. These changes may be required for the enhanced growth response induced by colonization of Trichoderma following seed or soil treatments. Stress and defense related proteins that were upregulated probably are related to the enhanced resistance conferred by the Trichoderma inoculation. We suggest that Trichoderma induces both increased growth, which is mediated by an increase in photosynthetic and respiratory rates, and systemic induced resistance. These two general effects may be mediated by different elicitors.
Addendum to: Shoresh M, Harman GE. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: A proteomic approach. Plant Physiol 2008; In press.
Trichoderma spp. have been known for decades to increase plant growth, shoot and root biomass and crop yield as well as to control numerous plant pathogens. When added as a seed or soil treatment, T. harzianum strain T22 colonizes roots and induces large changes in the shoot proteomes of maize seedlings (Zea mays) even though T22 is present only on roots.Citation1 A large portion of the upregulated shoot proteins was involved in carbohydrate metabolism and some proteins were related to photosynthesis. Increased photosynthesis should have resulted in increased starch accumulation in seedlings, and this did indeed occur.Citation1 Upregulation of carbohydrate metabolism and photosynthesis probably is required for the energy to drive the enhanced growth response conferred by root colonization of Trichoderma spp.Citation2 In addition, stress and defense related proteins were also induced and probably are essential for the induced systemic resistance response induced by the fungi.Citation2
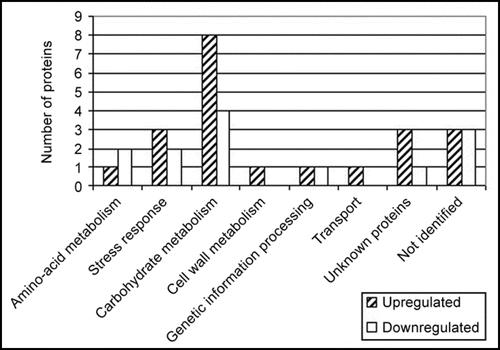
In roots, differential protein expression was less than in shoots. The number of proteins identified in shoots with differential expression between T22 inoculated and non-inoculated plants was 164, while in roots 31 proteins were found to be differentially expressed. In roots, as in shoots, the most numerous differentially expressed proteins were those involved in carbohydrate metabolism (). The changes in protein expression in shoots are probably due to translocated signals from the roots since the Trichoderma spp. are not present on the shoots. The greater changes in differential proteins in shoots as opposed to roots indicate a coordinated overall effect upon plant physiology. The coordinated system of changes probably is required for the overall beneficial effects upon plant growth and disease resistance that is noted as a consequence of plant colonization by these beneficial fungi.
These results begin to explain a paradox that has long been present when comparing our microbial-based systems of induction of resistance to more classical chemical induced systems. The consideration is that when systemic resistance is turned on, a metabolic cost is incurred that should result in reduced growth of plants. This is indeed true when materials such as flagellin 22, a bacterial elicitor of induced resistance, are examined. Other studies indicate a metabolic cost to the plant if disease resistance is induced by chemical elicitors.Citation3 This cost is intuitively obvious: since a series of proteins and metabolites are produced, there is a cost in energy and diversion of resources to these defense products. However, this is contrary to our understanding of the effects of microbial root symbionts that induce resistance. Rather than reducing growth, Trichoderma, Pseudomonas and Bacillus typically either have no effect on growth, or in some cases, substantially increase plant growth.Citation4 There are two explanations for a lack of negative effect on growth. First, some organisms, including at least T. asperellum and some Pseudomonas spp., exert their protective ability through a priming effect.Citation2,Citation5 In these cases, the organisms do not activate the defense-related genes, or do so only transitorily, but plants are primed to respond to pathogen attack through an earlier and stronger defense reaction once infection occurs. In cases studied thus far, it is usually the ISR pathway that is activated. Thus, there is little or no energy cost to the plant, other than the nutrition of the root-colonizing microbes, until pathogen attack occurs.
This priming effect, however, is not universally the case. In one study where disease resistance was systemic, a PR protein continued to be expressed.Citation6 In our studies with T. harzianum strain T22 as a seed treatment, the seedlings that emerged expressed numerous maize defense proteins that were not present in untreated plants.Citation1,Citation7 Thus, the defense genes in this case are turned on even in the absence of pathogens and so the priming effect is minimal or absent. The same observation was made with maize or cotton seedlings whose roots were colonized by T. virens.Citation8–Citation9 This activation of defense genes in maize seedlings treated with T22 occurs even though growth of the seedlings is markedly enhanced over control seedlings. In our proteomicCitation1 and transcriptomic studies (unpublished results), genes and proteins involved in energy metabolism, via both glycolysis and the TCA cycle, and photosynthesis are upregulated as well as PR products. These data suggest that T22 enhances plant growth at least in part because respiratory systems are upregulated and thereby increase energy to the growing plant. This, of course, cannot long be maintained unless the energy-producing systems, i.e., photosynthesis, are also upregulated. These results are consistent with the effects of T22 in the field.Citation4
In a study with tomato plants inoculated with Trichoderma hamatum, the expression of stress, cell wall and RNA metabolism related genes were also upregulated demonstrating similarities of plant responses to T. harzianum.Citation10 However, in this system carbohydrate metabolism related genes were not upregulated and no positive growth response was recorded. This strengthens our suggestion that there is a direct connection between the ability of Trichoderma to induce energy metabolism and its ability to induce growth response.
Thus, in plants whose roots are colonized by select strains of TrichodermaCitation4,Citation11,Citation12, BacillusCitation13 or PseudomonasCitation14, two separate general responses occur following successful root inoculations. The first is systemic disease resistance responses, which may occur either as a priming effect or via direct and continuous expression of PR genes and proteins or a combination of both. The second is a generalized increased growth response that may require increasing energy production. This logically requires enhanced respiratory rates to produce energy and also increased photosynthesis to support the greater respiratory rate.
While the mechanisms and elicitors for enhanced resistance are becoming known, the signaling and elicitation of the generalized increased growth phenomena are almost totally unknown. It is also not clear how these two induced responses are connected. It will therefore be very interesting to see future developments in this field regarding identification of elicitors and signal transducers of plant growth.
Figures and Tables
Acknowledgments
This research was supported in part by the US-Israel Agricultural Research and Development fund (BARD) grant US-3507-04 R and by Advanced Biological Marketing (Van Wert, OH). We thank Kristen Ondik for review and comments on the article.
Addendum to:
References
- Shoresh M, Harman GE. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: A proteomic approach. Plant Physiol 2008; 147:2147 - 2163
- Shoresh M, Yedidia I, Chet I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005; 95:76 - 84
- Dietrich R, Ploss K, Heil M. Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell Environm 2006; 28:211 - 222
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2004; 2:43 - 56
- van Loon LC. Plant responses to plant growth-promoting rhizobacteria. Eur J Plant Pathol 2007; 119:243 - 254
- Segarra G, Casanova E, Bellido D, Odena MA, Oliveira E, Trillas I. Proteome, salicylic acid and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum T34. Proteomics 2007; 7:3943 - 3952
- Shoresh M, Harman GE. Genome-wide identification, expression and chromosomal location of the genes encoding chitinolytic enzymes in Zea mays. Mol Genet Genom 2008; In press
- Djonovic S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Molec Plant Microbe Interact 2006; 8:838 - 853
- Djonovic S, Vargas WA, Kolomiets MV, Horndeski M, Weist A, Kenerley CM. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for systemic resistance in maize. Plant Physiol 2007; 145:875 - 889
- Alfano G, Lewis Ivey ML, Cakir C, Bos JIB, Miller SA, Madden LV, Kamoun S, Hoitink HAJ. Systemic modulation of gene expression in tomato by Trichoderma harzianum 382. Phytopathology 2007; 97:429 - 437
- Harman GE. Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis 2000; 84:377 - 393
- Harman GE, Petzoldt R, Comis A, Chen J. Interactions between Trichoderma harzianum strain T22 and maize inbred line Mo17 and effects of these interactions on diseases caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology 2004; 94:147 - 153
- Kloepper JW, Ryu CM, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004; 94:1259 - 1266
- van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 1998; 36:453 - 483