Abstract
Cucumber Mosaic Virus (CMV) is a highly infectious cucumovirus, which infects more than 800 plant species and causes major diseases in greenhouse and field crops worldwide. Parasitic weeds such as Phelipanche aegyptiaca are a major constraint to the production of many crops in the world and the parasite's lifestyle makes control extremely difficult. The parasite seeds can germinate after conditioning and perceiving strigolactones secreted by the host roots. Strigolactones are rhizosphere signaling molecules in plants that are biosynthesized through carotenoid cleavage. In the present study we investigated the possibility of reducing β-carotene and then strigolactone production in the host roots by blocking carotenoid biosynthesis using CMV-infected tobacco. It was found that CMV downregulated the enzyme phytoene desaturase(PDS) and reduced significantly both carotenoid production and Phelipanche infection in tobacco host roots infected with both CMV and P. aegyptiaca. Based on our results (decrease of β-carotene and repression of PDS transcripts in tobacco roots), we hypothesized that the reduction of Phelipanche tubercles and shoots occurred due to an effect of CMV on secondary metabolite stimulators such as strigolacetones. Our study indicated that mass production of the host roots was not affected by CMV; however, most inflorescences of Phelipanche grown on CMV-infected tobacco developed abnormally (deformed shoots and short nodes). Carotenoid biosynthesis inhibitors such as CMV can be used to reduce the production of strigolactones, which will lead to decreased Phelipanche attachment. Interestingly, attenuated CMV strains may provide a safe means for enhancing crop resistance against parasitic weeds in a future plan.
Introduction
Parasitic weeds such as Phelipanche aegyptiaca (Broomrapes) are obligate holoparasites that attack the roots of almost all economically important crops in semiarid regions of the world.Citation1-3 Parasitic weeds adopt different forms of invading host plants. Some invade aerial parts others invade the roots to obtain their nutrition for survival.Citation4,5 Broomrapes have developed sophisticated systems for detecting the presence of host plants and coordinating their development with the hosts.Citation6,4,7,8 The early stages of development are critical to parasite survival, because a germinated seedling that fails to connect to a host will exhaust its energy reserves and die. Some broomrape seeds compensate for this by strict protocols for germination and contact with the host. The first interaction between host and parasite is the induction of germination of the parasite by the host-root exudates (germination stimulants).Citation7,9 These exudates are secondary metabolites, generally produced in low quantities by the hosts (and some non-hosts). Once this step is fulfilled, the germinating parasite produces a radicle that must contact a host root and establish a connection. The third step requires a haustorium initiation factor, which causes the radicle tip to re-differentiate into a haustorium that penetrates the host root.Citation10 This organ forms the physical and physiological connection between parasite and host and its interaction with host tissues is important for translocation of molecules and macromolecules. By developing a strong metabolic sink in relation to the host, broomrape channels the flow of water and nutrients from the host to the parasite, thereby damaging crop development and tremendously reducing yields.Citation11 A single plant can produce hundreds of thousands of seeds, which can remain viable in the soil for over 10 y.Citation2,3,12 Phelipanche seed germination requires the presence of strigolactones exuded by the host plant.Citation9 It was demonstrated that strigolactones are the major class of germination stimulants and are derived from carotenoids through two subsequent enzymatic cleavage steps performed by the carotenoid cleaving dioxygenases CCD7 and CCD8.Citation13,14 Carotenoids are a large family of isoprenoid compounds that provide attractive natural pigments and coloration to flowers and fruits.Citation15 Carotenoids are photosynthesized exclusively by plants and some microorganismsCitation16 and their biosynthesis in plants occurs within the plastids, chloroplasts and chromoplasts of fruits and flowers.Citation17 Their importance as light sensors for photosynthesis, preventing photo-oxidation, and as nutritional components is also well recognized, because specific carotenoids (mainly β-carotene, α-carotene, and β-cryptoxanthin) are precursors of vitamin A which have significant antioxidant activity.Citation18 Carotenoids constitute an important precursor reservoir for the biosynthesis of bioactive compounds in plants, bacteria, fungi and even animals. Carotenoids are cleaved into apocarotenoids (also called norisoprenoids) by region-specific oxidative enzymes targeting different double bonds on the carotenoid backbone.Citation19-21 β-Carotene is classified as a terpenoid and is biosynthesized from lycopene after cyclization.Citation22 It is made up of eight isoprene units, which are cyclades at each end, and has nine conjugated double bonds. Cucumber Mosaic Virus (CMV) has a broad host range, which includes more than 800 plant species, and is transmitted by aphids in a non-persistent manner. CMV virions are isometric particles 30 nm in diameter. The CMV genome is organized into three single-stranded genomic RNAs (RNAs 1, 2, and 3) and two major subgenomic RNAs (RNAs 4 and 4A). RNAs 1 and 2 code for components of the replicase complex. RNA 2 also codes for the 2b protein (4A), which is involved in the suppression of gene silencing. RNA 3 encodes the 3a protein, which is essential for virus movement. The coat protein (24 kDa), is expressed from subgenomic RNA 4.Citation23 Plant viruses move from cell to cell via cellular connections (plasmodesmata) in an active process that is mediated by specialized virus-encoded movement proteins.Citation24 Some viruses can move as ribonucleoprotein complexes, other viruses (i.e.CMV) probably move as assembled virions, in which the coat protein is essential for the virus's long distance movement.Citation25 In this regard, it was shown that CMV can also move from virus-infested tomato plants to Phelipanche aegyptiaca tubercles and shoots attached to the virus-infested plants.Citation26 In the present study we show that the biosynthesis of the carotenoids can be inhibited through plant infection with CMV. We hypothesized that strigolactone concentration in tobacco root exudates would decrease, which would finally lead to reduced Phelipanche infection on the host plant.
Materials and Methods
Plant, parasite and virus materials
P. aegyptiaca Pers. seeds were collected from an infested tomato field in the South Galilee, Israel and used to infect tobacco host plants. Tobacco (Nicotiana tabacum L. cultivars Samsun NN) plants that served as host plants were maintained in a temperature-controlled greenhouse at 25°C with supplementary lighting.
Virus strain: Cucumber Mosaic Virus (Fny-CMV)Citation27 was used for the current study and maintained in tobacco (N. tabacum L., cultivars Samsun NN) seedlings as CMV inocula.
Host inoculation with both CMV and the parasite: Tobacco seedlings (four to five leaves) were transplanted into 0.5l pots filled with commercial soil. Tobacco plants were mechanically inoculated with CMV extract by rubbing the leaves (from plants with three to four leaves) after dusting with carborundum. One week later, the seedlings were transplanted into 10 l pots filled with soil (light-medium clay with 63% sand, 12% silt and 22% clay) artificially inoculated with P. aegyptiaca seeds (40 mg kg−1 soil). The pots were watered as needed and fertilized once e with 5 g Osmocote slow-release fertilizer (Scotts-Sierra Horticultural Products). Theplants were kept in a greenhouse under ambient sunlight at a temperature of 22 ± 4°C.
Assays for resistance to P. aegyptiaca
The plants were evaluated for resistance to P. aegyptiaca using an inoculated soil system. The experimental design was a randomized complete block with 30 replications for each treatment, and the experiment was repeated twice. Each week, starting at 28 d after P. aegyptiaca inoculation, five tobacco plants were removed from the pots and their roots were washed carefully to remove soil in order to determine parasite numbers, fresh weight of host roots and carotenoid content for each plant. Tobacco shoot dry weights were determined after drying for 24 h at 80°C. CMV-infected tobacco plants were compared with non-infected tobacco plants using Student's t test.
Carotenoid analyses
Carotenoids were extracted by grinding tobacco roots (1 gr.) in hexane: acetone: EtOH (2:1:1 v/v), followed by 5 min of saponififcation in 8% (w/v) KOH. The saponififed material was extracted twice with hexane, which was then evaporated in vacuum. The solid pellet was suspended in 400 μl of MeCN:MeOH:CH2Cl2 (45:5:50v/v) and passed through a 0.2 μm Nylon fiflter before HPLC analyses.Citation28 Samples for carotenoid extraction were pooled from frozen fresh roots of five tobacco plants.
HPLC analyses
HPLC analysis was performed according to Yahyaa et al. (2013).Citation28 Briefly, 40 microliters of fifltered fresh root extracts were injected into a 2996 Waters HPLC equipped with a Waters PDA detector 996, C18 Nova-Pak (Waters, Milford, MA, USA) column (250 × 4.6mm i.d.; 4 mm), and a Nova-Pak Sentry Guard cartridge (Waters, Milford, MA, USA). Detection was performed between 260 and 600nm. Data were analyzed using the MILLENIUM software.
RNA extraction and RT-PCR of the phytoene desaturase (PDS) gene
Total RNA was extracted from tobacco roots (pooling material from five different tobacco plants) 45 d after infection with the parasite. RNA was isolated using the TRI-REAGENT kit (Molecular Research Center, Inc., Cincinnati, OH, USA), according to the manufacturer's instructions. RNA concentrations were measured using a spectrophotometer (Nanodrop ND-1000, Wilmington, DE, USA) and comparable amounts of the different RNAs were subjected to RT-PCR analysis. Total RNA (1 μg) was reverse-transcribed with an oligo dT primer using Verso cDNA Kit (ABgene, Epsom UK) according to the manufacturer's instructions. The resulting cDNA was used as a template in a PCR to amplify the PDS gene using the PDS-specific primers 5′GTG AAA CAC AAC TCC TTG GC -3′ (forward) and 5′- TCC CTA GTT CTC CAA ACA GG -3′ (reverse). PCR conditions consisted of 94°C for 4 min, 35 cycles of 94°C for 1 min, 48°C for 30 s, 72°C for 1 min, and a 72°C final extension for 7 min. The expected 550 bp product was visualized on a 0.8% gel stained with ethidium bromide. Actin expression was used as an internal control for PCR amplification of the resultant cDNA with the specific forward primer: 5′-AATGATCGGAATGGAAGCTG -3′; and the specific reverse primer: 5′-TCCACTGAAGGACGA TGTTTC-3′.
Results and Discussion
Although several compounds from different secondary metabolite classes have been reported to be secreted by the host root and have germination stimulating activity, Phelipanche seed germination required the presence of strigolactones exuded by the host plant.Citation9 Strigolactones, the major class of germination stimulants,Citation14 are derived from carotenoids through two subsequent enzymatic cleavage steps performed by the carotenoid cleaving dioxygenases CCD7 and CCD8.Citation29-32 To date, the impact of virus-infected host plant on parasitism by Phelipanche has not been reported, therefore our study focused on carotenoid content in CMV-infected tobacco roots and its effect on the development of the parasite.
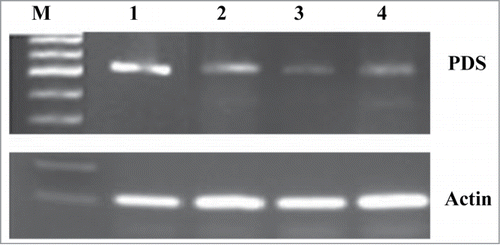
To investigate whether CMV affects carotenoid production in CMV-infected tobacco plants, the β-carotenecontent in the roots was measured each week of the host's growth period (28–63 d). β-Carotene content was significantly affected by CMV infection when tobacco plants were infected only with the virus (). A slight reduction in β-carotene content was observed when tobacco plants were infected with Phelipanche or with both CMV and Phelipanche. However, CMV-infected tobacco alone significantly reduced the concentration of β-carotene by 50% compared with uninfected tobacco roots (). Previously, it has been reported that biochemical changes in CMV-infected Amaranthus and Chenopodium were shown.Citation33 Carotenoids were found to be highly sensitive to CMV infection, their levels decreased drastically (19–52%), followed Amaranthus and Chenopodium infected leaves.Citation33 Jamil et al. (2010)Citation34 reported that irrigation application of fluridone, norflurazon and clomazone (inhibitors of carotenoid biosynthesis) to rice significantly reduced the concentration of known strigolactones in host root exudates causing reduction of Striga infection in rice. Pepino Mosaic Virus (PepMV) is a highly infectious potexvirus and a major disease of greenhouse tomato crops worldwide. It was shown that all genes encoding enzymes involved in the biosynthesis of lycopene, including geranylgeranyl pyrophosphate synthase, phytoene synthase, phytoene desaturase, z-carotene desaturase, and carotene isomerase, were suppressed upon PepMV infection in tomato seedlings.Citation35 To study transcriptional gene changes involved in the biosynthesis of β-carotene in response to CMV infection, we tested the effect of CMV on the accumulation of phytoene desaturase (PDS) transcripts, a key-enzyme in β-carotene biosynthesis in tobacco plants. Total RNA from tobacco roots infected with P. aegyptiaca or from tobacco roots infected with both P. aegyptiaca and CMV was extracted 45 d after infection (dpi) and subjected to RT-PCR using specific primers corresponding to PDS and actin genes. PDS RNA was detected in all of the examined treatments. However, CMV inoculation induced a different transcriptomic response, which resulted in repression of PDS transcripts in tobacco roots infected with CMV rather than inuninfected tobacco (). Our present study suggests that PDS biosynthesis in tobacco roots is transcriptionally regulated, and that CMV affects carotenoid accumulation and gene expression in the infested tissue. Recent findingsCitation36 reported that phytoene synthase and 1-deoxy-D-xylulose-5-phosphate synthase genes are the main gene members controlling carotenoid biosynthesis in citrus fruit. We assume that the PDS gene also has an important role in controlling carotenoids in tobacco plants.
Figure 1. Inhibition of β-carotene production in CMV-infected tobacco roots. Bars represent means of peak areas ± SE of the individual β-carotene as determined by HPLC in triplicate. Data were analyzed using JMP® software (version 4.0.3, SAS Institute Inc.).
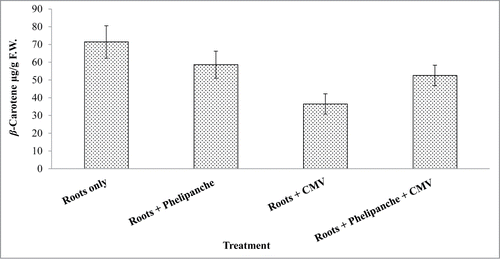
Figure 2. Evaluation of PDS gene expression in tobacco roots following infection with P. aegyptiaca and CMV. Gene-specific RT-PCR detection of PDS mRNA (upper part) from tobacco roots (line 1), tobacco roots infected with P. aegyptiaca (line 2), tobacco roots infected with CMV alone (line 3), tobacco roots infected with both CMV and P. aegyptiaca (line 4). Expression of an endogenous actin gene (159 bp) (lower part) was used as a positive control for RNA quality for all RNA samples.
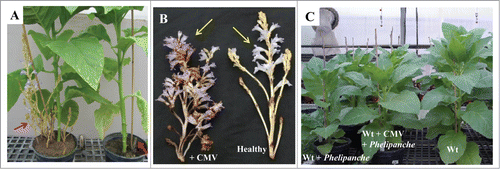
In order to evaluate the effect of CMV on host resistance to parasitism by Phelipanche, experiments were conducted in soil which more closely approximated field conditions. In this study it has been observed that the number and biomass of the parasite tubercles (10–45 mg fresh weight/tubercle) and shoots were significantly lower in tobacco plants infected by both CMV and the parasite as compared with those which developed on tobacco plants infected by the parasite alone (, ). In this study, it has been observed that most CMV-infected tobacco plants developed deformed parasite shoots with short nodes, which were not observed in the Phelipanche inflorescences grown on healthy host plants (). In an earlier study, CMV-infected dodder grown on tobacco showed similar abnormalities.Citation37 Tobacco leaves infected with CMV showed a variety of symptoms. Some infected leaves showed typical mosaic symptoms; others did not display any visible symptoms. Additionally, we observed that the height of CMV-infected tobacco plants was not affected by the presence of the parasitic plant and the resulting plants appeared normal and were fertile (). We assume that the normal height of CMV-infected plants was caused by the fact that less infection was recorded by the parasite in these plants. However, the number of Phelipanche shoots per plant was significantly higher in uninfected tobacco than in CMV-infected plants 45 d post-inoculation (dpi). However, we observed that 65 dpi more parasitic shoots developed in CMV-infected tobacco plants, indicating a delay in the appearance of P. aegyptiaca on CMV-infected tobacco plants.
Figure 3. Effect of CMV-infected tobacco plants on P. aegyptiaca parasitization. Numbers of P. aegyptiaca (tubercles and shoots) parasitizing tobacco plants (bright columns), and numbers of the parasites parasitizing tobacco plants infected with both CMV and the parasite (dark columns). Bars represent means of 10 replicates and vertical lines indicate SE. Data were analyzed using JMP® software (version 4.0.3, SAS Institute Inc.). Averages were compared using Student's t test (with α = 0.05)
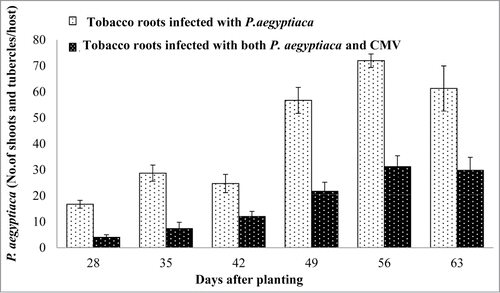
Figure 4. Development of the parasite Phelipanche aegyptiaca on CMV- infected Nicotiana tabacum L. (cultivars Samsun NN) and on healthy tobacco plants. (A) Nicotiana tabacum L. (left) is an uninfected plant and (right) is a CMV-infected Nicotiana tabacum L. plant. Arrows indicate Phelipanche aegyptiaca shoots. (B) Representative inflorescences of P. aegyptiaca grown on tobacco infected with CMV (left) and on control healthy tobacco (right). Pictures were taken 65 d after inoculation with Phelipanche seeds in a pot system. (C) Effect of CMV on the height of tobacco plants as compared with uninfected wild type (wt) plants.
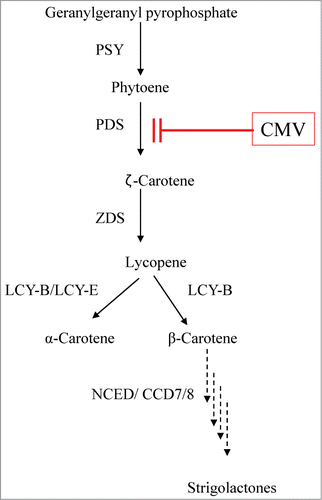
Based on the current results (decrease of β-carotene and repression of PDS transcripts in tobacco roots), we hypothesize that the reduction in the number of Phelipanche tubercles and shoots was due to an effect of CMV on secondary metabolite stimulators such as strigolacetones, which are produced via the carotenoid biosynthesis pathway.Citation38,30,39 In fact, in this study it was found that phytoene content in the host roots increased following CMV infection (data not shown). Therefore, we postulate that CMV block PDS catalyzes the conversion of phytoene to phytofluene (). This could lead to inhibition of β-carotene biosynthesis particularly in the roots, and eventually could lead to a decrease in the production of strigolactones. This may result in decreased germination of the parasite seeds and hence in reduced P. aegyptiaca infection.
Figure 5. Effect of CMV-infected tobacco plants on the development of tobacco roots in a soil-based assay. Fresh weight measurement of tobacco host roots infected with P. aegyptiaca (bright columns) compared with host roots infected with both P. aegyptiaca and CMV (dark columns). Bars represent means of 10 replicates and vertical lines indicate SE. Asterisks above columns indicate a statistically significant effect between treatments. Data were analyzed using JMP® software (version 4.0.3, SAS Institute Inc.). Averages were compared using Student's t test (with α = 0.05).
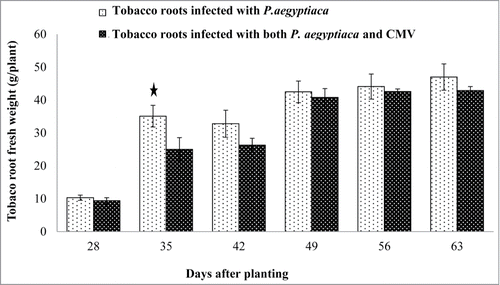
Figure 6. Schematic diagram showing carotenoid biosynthetic pathway in N. tabacum and site of action of CMV as inhibitor of PDS. PSY, phytoene synthase; PDS, phytoene desaturase; ZDS, ζ-carotene desaturase; LCY-B, β-cyclase; LCY-E, ϵ-cyclase; CCD, carotenoid cleavage dioxygenase; NCED, 9-cis-epoxycarotenoid dioxygenase; CMV block phytoene desaturase (PDS). The arrows represent enzymatic steps.
The infection rate of tobacco host plants by P. aegyptiaca could be affected by the biomass of the host roots. To exclude the effect of CMV on the biomass of tobacco roots, the fresh weight of tobacco host roots infected with P. aegyptiaca was compared with the fresh weight of host roots infected with both P. aegyptiaca and CMV during the host's growth period (). To quantify this observation, each week tobacco roots were pooled from three plants and weighed. As shown in , no significant differences were observed in the host root biomass. For example, after 63 d, the fresh weight of tobacco host roots infected with P. aegyptiaca was 47 g as compared with 43 g in tobacco infected with both P. aegyptiaca and CMV.
In summary, carotenoid biosynthesis inhibitors such as CMV can be used to reduce the production of strigolactones () and their secretion into the rhizosphere, which may result in decreased broomrape attachment. Interestingly, attenuated CMV strains in which no severe phenotypic and developmental impairment were elicited by the wild type CMV could possibly provide a safe means for enhancing crop resistance against parasitic weeds.
Further study is needed to clarify the percentage of strigolactone reduction in the host roots following CMV infection.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge Ms. Ayala Meir of the Department of Plant Genetics, ARO, Newe Ya’ar Research Centre, Ramat Yishay, Israel for her assistance in HPLC analysis of carotenoids.
Funding
The results of this research were supported by the Chief Scientist of the Ministry of Agriculture and Rural Development, Israel, grant No.132–1499–10 (MEZAM ALEKET). The authors also acknowledge STREAM, COST Action FA1206 for their invitation to present our results through activity of WG2 (Strigolactones as signals for parasitic plants).
References
- Parker C, Riches CR. Parasitic weeds of the world: Biology and Control. Wallingford: CAB International, 1993.
- Parker C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag Sci 2009; 65:453-9; PMID:19206075; http://dx.doi.org/10.1002/ps.1713
- Parker C. Parasitic Weeds: A World Challenge. Weed Sci 2012; 60:269-76; http://dx.doi.org/10.1614/WS-D-11-00068.1
- Yoder JI. Parasitic plant responses to host plant signals: a model for subterranean plant-plant interactions. Curr Opin Plant Biol 1999; 2:65-70; PMID:10047574; http://dx.doi.org/10.1016/S1369-5266(99)80013-2
- Joel DM, Hershenhorn J, Eizenberg H, Aly R, Ejeta G, Rich PJ, et al. Biology and management of wedy root parasites. Horticultural eviews: John Wiley & Sons, Inc., 2007:267-349.
- Joel D, Steffens J, Matthews D. Germination of weedy root parasites. Seed development and germination 1995; 1.
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH. Secondary metabolite signalling in host-parasitic plant interactions. Curr Opin Plant Biol 2003; 6:358-64; PMID:12873531; http://dx.doi.org/10.1016/S1369-5266(03)00065-7
- Aly R. Conventional and biotechnological approaches for control of parasitic weeds. In Vitro Cell Dev-Pl 2007; 43:304-317.
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007; 225:1031-8; PMID:17260144; http://dx.doi.org/10.1007/s00425-006-0410-1
- Keyes WJ, Taylor JV, Apkarian RP, Lynn DG. Dancing together. Social controls in parasitic plant development. Plant Physiol 2001; 127:1508-12; PMID:11743095; http://dx.doi.org/10.1104/pp.010753
- Joel DM. The long-term approach to parasitic weeds control: manipulation of specific developmental mechanisms of the parasite. Crop Prot 2000; 19:753-8; http://dx.doi.org/10.1016/S0261-2194(00)00100-9
- Parker C, Riches CR. Parasitic Weeds of the World: Biology and Control. 1993.
- Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 2005; 139:920-34; PMID:16183851; http://dx.doi.org/10.1104/pp.105.061382
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 2007; 12:224-30; PMID:17416544; http://dx.doi.org/10.1016/j.tplants.2007.03.009
- Hirschberg J. Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 2001; 4:210-8; PMID:11312131; http://dx.doi.org/10.1016/S1369-5266(00)00163-1
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res 2007; 55:207-16; PMID:17349800; http://dx.doi.org/10.1016/j.phrs.2007.01.012
- Datta K, Baisakh N, Oliva N, Torrizo L, Abrigo E, Tan J, Rai M, Rehana S, Al-Babili S, Beyer P, et al. Bioengineered ‘golden’ indica rice cultivars with beta-carotene metabolism in the endosperm with hygromycin and mannose selection systems. Plant Biotechnol J 2003; 1:81-90; PMID:17147745; http://dx.doi.org/10.1046/j.1467-7652.2003.00015.x
- Kato M, Ikoma Y, Matsumoto H, Sugiura M, Hyodo H, Yano M. Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol 2004; 134:824-37; PMID:14739348; http://dx.doi.org/10.1104/pp.103.031104
- Auldridge ME, McCarty DR, Klee HJ. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 2006; 9:315-21; PMID:16616608; http://dx.doi.org/10.1016/j.pbi.2006.03.005
- Walter MH, Floss DS, Strack D. Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 2010; 232:1-17; PMID:20396903; http://dx.doi.org/10.1007/s00425-010-1156-3
- Walter MH, Strack D. Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep 2011; 28:663-92; PMID:21321752; http://dx.doi.org/10.1039/c0np00036a
- Schofield A, Paliyath G. Modulation of carotenoid biosynthesis during tomato fruit ripening through phytochrome regulation of phytoene synthase activity. Plant Physiol Biochem 2005; 43:1052-60; PMID:16442806; http://dx.doi.org/10.1016/j.plaphy.2005.10.006
- Palukaitis P, Roossinck MJ, Dietzgen RG, Francki RI. Cucumber mosaic virus. Adv Virus Res 1992; 41:281-348; PMID:1575085; http://dx.doi.org/10.1016/S0065-3527(08)60039-1
- Hosford RMJ. Transmission of plant viruses by dodder. Bot Rev 1967; 42:387-406; http://dx.doi.org/10.1007/BF02858742
- Oparka KJ, Roberts AG. Plasmodesmata. A not so open-and-shut case. Plant Physiol 2001; 125:123-6; PMID:11154313; http://dx.doi.org/10.1104/pp.125.1.123
- Gal-On A, Naglis A, Leibman D, Ziadna H, Kathiravan K, Papayiannis L, Holdengreber V, Guenoune-Gelbert D, Lapidot M, Aly R. Broomrape can acquire viruses from its hosts. Phytopathology 2009; 99:1321-9; PMID:19821737; http://dx.doi.org/10.1094/PHYTO-99-11-1321
- Rizzo TM, Palukaitis P. Construction of full-length cDNA clones of cucumber mosaic virus RNAs 1, 2 and 3: generation of infectious RNA transcripts. Mol Gen Genet 1990; 222:249-56; PMID:2274028; http://dx.doi.org/10.1007/BF00633825
- Yahyaa M, Bar E, Dubey NK, Meir A, Davidovich-Rikanati R, Hirschberg J, Aly R, Tholl D, Simon PW, Tadmor Y, et al. Formation of norisoprenoid flavor compounds in carrot (Daucus carota L.) roots: characterization of a cyclic-specific carotenoid cleavage dioxygenase 1 gene. J Agric Food Chem 2013; 61:12244-52; PMID:24289159; http://dx.doi.org/10.1021/jf404085k
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. Strigolactone inhibition of shoot branching. Nature 2008; 455:189-94; PMID:18690209; http://dx.doi.org/10.1038/nature07271
- Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 2012; 335:1348-51; PMID:22422982; http://dx.doi.org/10.1126/science.1218094
- Cheng X, Ruyter-Spira C, Bouwmeester H. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front Plant Sci 2013; 4:199; PMID:23785379
- Aly R, Dubey N, Yahyaa M, Abu-Nassar J, Ibdah M. Gene silencing of CCD7 and CCD8 in Phelipanche aegyptiaca by tobacco rattle virus system retarded the parasite development on the host. [Epub ahead of print]. Plant Signal Behav 2014; 9:e29376; PMID:24874436; http://dx.doi.org/10.4161/psb.29376
- Prakash D, Raj SK, Singh BP. Biochemical-changes in cucumber mosaic-virus (CMV)-infected amaranthus and chenopodium. J Sci Food Agric 1995; 68:299-303; http://dx.doi.org/10.1002/jsfa.2740680307
- Jamil M, Charnikhova T, Verstappen F, Bouwmeester H. Carotenoid inhibitors reduce strigolactone production and Striga hermonthica infection in rice. Arch Biochem Biophys 2010; 504:123-31; PMID:20732294; http://dx.doi.org/10.1016/j.abb.2010.08.005
- Hanssen IM, van Esse HP, Ballester A-R, Hogewoning SW, Parra NO, Paeleman A, Lievens B, Bovy AG, Thomma BP. Differential tomato transcriptomic responses induced by pepino mosaic virus isolates with differential aggressiveness. Plant Physiol 2011; 156:301-18; PMID:21427280; http://dx.doi.org/10.1104/pp.111.173906
- Peng G, Wang C, Song S, Fu X, Azam M, Grierson D, Xu C. The role of 1-deoxy-d-xylulose-5-phosphate synthase and phytoene synthase gene family in citrus carotenoid accumulation. Plant Physiol Biochem 2013; 71:67-76; PMID:23883976; http://dx.doi.org/10.1016/j.plaphy.2013.06.031
- Costa AS. Multiplication of virus in dodder, Cuscuta camestrls. Phytopathology 1944; 34:151-62.
- López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, Verstappen F, Puech-Pages V, Bécard G, Mulder P, et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 2008; 178:863-74; PMID:18346111; http://dx.doi.org/10.1111/j.1469-8137.2008.02406.x
- Kohlen W, Charnikhova T, Lammers M, Pollina T, Tóth P, Haider I, Pozo MJ, de Maagd RA, Ruyter-Spira C, Bouwmeester HJ, et al. The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol 2012; 196:535-47; PMID:22924438; http://dx.doi.org/10.1111/j.1469-8137.2012.04265.x
