 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Recently we have suggested that (glucurono)arabinoxylans [(G)AX] feruloylation and oxidative coupling occur both intra-protoplasmically and, extra-protoplasmically, in the plant cell wall. In this work we illustrate a model of two possible mechanisms of polysaccharide feruloylation and oxidative coupling in plants. Moreover, we take into consideration the possible role of in muro feruloylation as a rapid defense mechanism against potential plant pathogen and parasite infections.
Oxidative burst is one of the fastest defense responses activated by plants in order to resist to pathogen and parasite attacks. It consists in the production of reactive oxygen species (ROS), mainly hydrogen peroxide (H2O2), at the first site of pathogen invasion: the plant cell wall. In the presence of ROS, a rapid formation of intermolecular cross-links among various polymers of the cell wall occurs with consequent increase of the resistance to pathogen penetration and spread. These events take place before the transcription-dependent defense mechanisms are activated.Citation1–Citation4
The hydroxycinnamic acids, in particular ferulic acid, ester-linked to arabinose and galactose of pectic and hemicellulosic polysaccharides, are thought to be the main cause of cross-link formation among cell wall structural polymers. It has been well established that oxidative coupling of polysaccharides through their ester-linked feruloyl groups occurs both within the protoplast and after secretion into the apoplast. This process is catalyzed by multiple cell wall bound and, possibly, Golgi resident isoforms of peroxidases which use hydrogen peroxide as substrate.Citation5,Citation6 The formation of different isomers of intra- and inter-polysaccharidic dehydrodimers and oligomers of ferulate is thought to contribute to wall assemblyCitation7 and to plant defense responses by restricting cell wall accessibility to lytic enzymes.Citation3 The site, or sites, of the transfer of feruloyl groups on to polysaccharides is, in contrast, still controversial. Although many pieces of evidence suggest that feruloylation occurs mainly intra-protoplasmically, during the de novo synthesis of matrix polysaccharides at the Golgi apparatus level,Citation7–Citation10 by comparing the kinetics of appearance of arabinosyl- and feruloyl-radiolabelled polysaccharides in the protoplasmic compartment and their secretion in the wall of wheat seedling root apical segments, we have recently suggested that an additional and more rapid feruloylation reaction may take place extra-protoplasmically on pre-existing polysaccharides.Citation11
Here we propose a graphic model () summarizing the two possible mechanisms of attachment of a feruloyl group to a polysaccharide chain and the oxidative coupling of feruloyl residues to form bridges. It comprises: (i) a mechanism by which feruloylation and feruloyl coupling occur within the protoplast, most likely in the Golgi apparatus;Citation7–Citation10 (ii) a mechanism that occurs in muro.Citation11–Citation13 In this model, the endogenous cinnamate synthesized in the cytoplasm from phenylalanine by the action of phenylalanine ammonia lyase (PAL), a key enzyme in the phenolic metabolism of plants, is rapidly converted to feruloyl-CoA thorough the phenylpropanoid pathway. The same conversion also occurs to the exogenously supplied [14C] cinnamate, which, at physiological pH, rapidly permeates through the plasma membrane in absence of a specific carrier. The feruloyl-CoA represents the major donor substrate from which feruloyl groups are transferred on to the arabinose residues of (glucurono) arabinoxylans [(G)AX] during their synthesis into the Golgi apparatus. As previously described, feruloyl-(G)AX can be oxidatively coupled within this compartment by forming ferulate dimers or larger coupling products. Feruloyl-polysaccharides and their coupled derivatives are transported into the wall by the secretion pathway, passing through the trans-Golgi network (TGN). This pathway is sensitive to Brefeldin A, a well-known inhibitor of secretion, and requires at least 5 minutes of transit time before the newly synthesized feruloyl-polysaccharides reach the cell wall.Citation11
Alternative feruloyl donors could be the glycosidic esters of ferulate, e.g., 1-O-feruloyl-β-D-glucose (β-Glc-Fer), or other still unknown activated feruloyl precursors (AFP). These compounds may be synthesized in the cytoplasm where they can act as a “reserve” pool of feruloyl units that may be gradually recruited for intracellular polysaccharide feruloylation, either directlyCitation9 or via a small and rapid turning-over pool of feruloyl-CoACitation14 and/or, in part, sequestered as vacuolar conjugates.Citation15 Besides, these low-Mr activated metabolites may be released from the protoplasm into the apoplast with a rapid (>2 min) and BFA insensitive mechanism. In cultured wheat cells, Obel et al.Citation9 have reported the release of a high proportion of the total β-Glc-Fer into the medium. In the apoplast these compounds could act as potential donor substrates of feruloyl residues for in muro O-esterification reactions of arabinosyl groups of pre-existing (G)AX catalyzed by new hypothetical extracellular feruloyl transferases.
Since it has been proposed that the majority of feruloyl groups that were suitably positioned to be capable of coupling had already dimerised intra-protoplasmically,Citation7 the rapid addition of feruloyl groups in muro could give new substrates for dimers formation to strengthen the plant cell wall during the first phases of pathogen infection. In the light of this hypothesis, we incubated 3-day-old wheat seedlings (Triticum durum Desf., cv. Capeiti) for 3 h in the presence of the H2O2-generating system glucose (0.5 mM)/glucose oxidase (0.5 Uml−1) (G/GO) that determine a steady-state accumulation of ∼6 µM H2O2 for a time longer than 3 h, closely mimicking the oxidative burst induced by a pathogen attack.Citation16 After 1 h of incubation in the presence or absence of G/GO, trans-[U-14C]cinnamic acid was added as a tracer. As previously reported,Citation11 the trans-[U-14C] cinnamic acid was rapidly taken up by the seedlings and metabolized in a series of intermediate molecules, including feruloyl-CoA and, probably, β-Glc-Fer that are used as substrate for the synthesis of feruloyl-polysaccharides. All the analyses were performed on 1 cm long apical root segments (1 cm) excised from the seedlings at the end of the incubation time as previously described.Citation11 Oxidative stress did not influence the uptake, but it led to a significant increase in the incorporation of 14C into the cell wall polymeric material (). Subjecting the destarched and partially deproteinated cell walls to saponification with diluted alkali, the ester-linkages were hydrolyzed liberating monomers of 14C-ferulic acid but also 14C-dimers, 14C-trimers and 14C-oligomers. These compounds were acidified, partitioned against ethyl acetate and partially resolved by TLC on Merck silica-gel F254 plates (plastic-backed, with fluorescent indicator) (Merck Sharp & Dohme S.p.A., Rome, Italy) in benzene/acetic acid (9;1, v/v; BzA) for 2.5 h as described by Fry et al.Citation14 By counting the radioactivity associated to each compound, we confirmed that 14C-trimers and products with higher molecular weight (RF 0.00 on TLC) are the major oxidative coupling products released by saponification of the wall fraction either in the presence or absence of G/GO, suggesting that they are the most involved in polysaccharide cross-linking and cell wall stiffening. In addition we found that, although a 100% increase in the cell wall polysaccharides ester-linked 14C-diferulates, 14C-triferulates and 14C-ferulic acid oligomers was clearly determined by the G/GO treatment, the amount of ester-linked ferulic acid monomers did not significantly decrease (). If polysaccharide feruloylation occurred exclusively inside the protoplast, a decrease of feruloyl monomers in favor of an increase of coupling products could be expected when the in muro oxidative coupling is stimulated by the extracellular production and accumulation of H2O2. The stable content of feruloyl monomers, even in condition of oxidative stress, is easily explicable considering the presence of an in muro addition of feruloyl groups as proposed by our model. The in muro feruloylation would increase polysaccharide degree of substitution and hence their capacity to dimerize. The amount of dehydrodimers is, in fact, proportional to that of esterified ferulic acid.Citation10 The in muro addition of feruloyl residues keeps the amount of monomers of ferulate ester-linked to cell wall polysaccharides stable. In conclusion, the rapid increase of the number of ferulic acid residues linked to the pre-existing polymeric components of the cell wall and on the level of cross-linking between the feruloyl-polysaccharides, which happens in muro to strengthen the wall, could represent a rapid defense response against potential plant pathogen infection.
Figures and Tables
Figure 1 Potential routes of (G)AX feruloylation and oxidative coupling. , arabinoxylan; F, feruloyl group; TGN, trans-Golgi network; BFA, Brefeldin A; AFP, Activated feruloyl precursor.
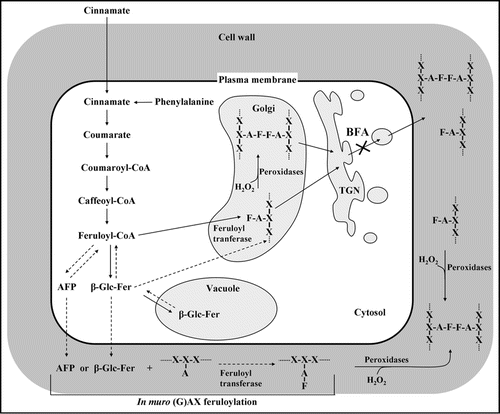
Table 1 Distribution of the radioactivity incorporated from trans-[U-14C]cinnamic acid into the polymers of the purified cell walls, defined by their extractability into pronase and 0.1 M NaOH
Table 2 Quantitative and qualitative composition of 14C-feruloyl derivatives ester linked to destarched and partially deproteinated purified cell walls
Acknowledgements
This work was financed by MIUR, project AGRO-GEN.
Addendum to:
References
- Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem J 1997; 322:681 - 692
- Grant JJ, Loake GJ. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 2000; 124:21 - 30
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, et al. The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 2002; 53:1367 - 1376
- Low PS, Merida JR. The oxidative burst in plant defense: Function and signal transduction. Physiol Plant 2006; 96:533 - 542
- Wallace C, Fry SC. In vitro peroxidase-catalysed oxidation of ferulic acid esters. Phytochem 1995; 39:1293 - 1299
- Fry SC. Oxidative coupling of tyrosine and ferulic acid residues: Intra- and extra-protoplasmic occurrence, predominance of trimers and larger products, and possible role in interpolymeric cross-linking. Phytochem Rev 2004; 3:97 - 111
- Lindsay SE, Fry SC. Control of diferulate formation in dicotyledonous and gramineous cell-suspension cultures. Planta 2008; 227:439 - 452
- Myton KE, Fry SC. Intraprotoplasmic feruloylation of arabinoxylans in Festuca arundinacea cell cultures. Planta 1994; 193:326 - 330
- Obel N, Porchia AC, Scheller HV. Intracellular feruloylation of arabinoxylan in wheat: evidence for feruloyl-glucose as precursor. Planta 2003; 216:620 - 629
- Philippe S, Tranquet O, Utille J-P, Saulnier L, Guillon F. Investigation of ferulate deposition in endosperm cell walls of mature and developing wheat grains by using a polyclonal antibody. Planta 2007; 255:1287 - 1299
- Mastrangelo LI, Lenucci MS, Piro G, Dalessandro G. Evidence for intra- and extra-protoplasmic feruloylation and cross-linking in wheat seedling roots. Planta 2009; 229:343 - 355
- Nishitani K, Nevins DJ. Enzymic analysis of feruloylated arabinoxylans (feraxan) derived from Zea mays Cell Walls III. Structural changes in the feraxan during coleoptile elongation. Plant Physiol 1990; 93:396 - 402
- Encina A, Fry SC. Oxidative coupling of a feruloyl-arabinoxylan trisaccharide (FAXX) in the walls of living maize cells requires endogenous hydrogen peroxide and is controlled by a low-Mr apoplastic inhibitor. Planta 2005; 223:77 - 89
- Fry SC, Willis SC, Paterson EJ. Intraprotoplasmic and wall-localised formation of arabinoxylan-bound diferulates and larger ferulate coupling-products in maize cell-suspension cultures. Planta 2000; 211:679 - 692
- Harborne JB, Corner JJ. Plant polyphenols 4. Hydroxycinnamic acid-sugar derivatives. Biochem J 1961; 81:242 - 250
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998; 394:585 - 588