Abstract
The rooting of stem cuttings is a common vegetative propagation practice in many ornamental species. Among other signals, auxin polarly transported through the stem plays a key role in the formation and growth of adventitious roots. Unlike in other plant species, auxin from mature leaves plays a decisive role in the rooting of carnation (Dianthus caryophyllus. L) cuttings. The gene DcAUX1, which codifies an auxin influx carrier involved in polar auxin transport, has now been cloned and characterized in carnation. The expression pattern of this gene was seen to depend on the organ, the cultivar and the time of cold storage. The variations observed in its expression could be related with the rooting ability of different carnation cultivars.
Ornamental plant production is an important part in the global market for agricultural products, among which carnation (Dianthus caryophyllus L.) is one of the most popular commodities.
The rooting of cuttings is the most common way of propagating carnation plants and many commercial companies offer cuttings of selected registered varieties just rooted or ready for rooting. However, as in the production of young plants of other species, carnation cuttings are not planted immediately after excision but are usually stored for several weeks to match production with demand. The postharvest protocol implies: (a) storage in a cold chamber to preserve the cuttings and (b) to planting the stored cuttings for them to root. Each step has its own particular features and while the exact influence of storage on rooting has hardly been investigated, the movement of signals from apical to basal zones has been the subject of intense research.
The rooting process is a complex sequence whereby, in a first phase, some cells take on a special status (stem cells) to divide around an organized centre, allowing the appearance of a meristem. In a second phase, cell growth will lead to a structured root appearing. Furthermore, in addition to other signals, auxin is involved in the rooting process,Citation1,Citation2 as deduced from the inhibition of rooting observed after removal of the presumed endogenous auxin source by decapitation and/or debudding, a process that is restored when exogenous auxin is added.Citation3,Citation4 Polar auxin transport (PAT) is necessary for the formation of adventitious roots, as demonstrated in several studies in which the application of PAT inhibitors such as naphthylphtalamic acid (NPA) and triiodobenzoic acid (TIBA) was seen to strongly inhibit the rooting of cuttings.Citation5,Citation6 Basipetal PAT in stems is found in the xylem parenchyma and involves specific auxin transport carriers, such as the influx (AUX1, LAX) and efflux (PIN1-8, P-glycoprotein (PGP)) auxin carriers that have been identified in several species.Citation7–Citation10
As indicated above, in the commercial production of rooted plants, cuttings are usually stored in a cold chamber for several weeks. The aim of our first studies, therefore, was to investigate the influence of cold storage and auxin treatment on the subsequent rooting of carnation cuttings. The results showed that cold storage alters rooting and might be as effective as auxin treatment in stimulating the formation and growth of adventitious roots. The effects were dependent on the length of storage and the carnation cultivar and suggested that changes in endogenous auxin might occur during storage.Citation11,Citation12 Later, we carried out experiments to search for the origin of the endogenous auxin responsible for the rooting. The resultsCitation13 showed that the suppression of the young leaves and/or the apex did not inhibit rooting, while the suppression of mature leaves strongly reduced rooting. The application of exogenous auxin directly to the rooting zone (i.e., the basal internode) of cuttings without mature leaves restored rooting. Moreover, after application of radioactive IAA to mature leaves, radioactivity was recovered in the rooting zone rather than in the apex. These results suggest that, unlike in other types of cuttings, where the auxin source for rooting is the apex or buds, mature leaves are the auxin source in carnation cuttings. The presence of a lanoline ring containing NPA in the basal internode of intact cuttings fully inhibited rooting and reduced the accumulation of radioactivity in the rooting zone after application of radioactive IAA to mature leaves. Once the role of PAT in the rooting of carnation cuttings had been confirmed, the features of PAT and the effects of its inhibitors were extensively studiedCitation14 and the PAT measurement method was improved. The influence of cold storage on PAT was also studied and a parallel variation in PAT and rooting was observed in some cultivars.Citation14,Citation15
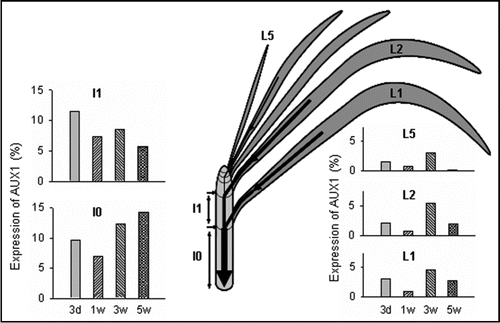
Unlike for lateral roots, there is almost no information on the molecular mechanism that controls PAT during the formation of adventitious roots, especially in cuttings used for vegetative propagation. To investigate the molecular basis of the PAT variations observed in carnation cuttings, we have searched for genes coding for putative auxin carriers. A novel cDNA encoding an auxin influx carrier was isolated and characterized. The full length of DcAUX1 (Access no. AM235386) was obtained and the aminoacid sequence revealed a high degree of identity with the corresponding auxin carrier proteins from several species.Citation16 The expression of this gene depends on the organ, the carnation cultivar and the length of time cuttings are stored in a cold chamber. As a rule, expression is higher in stem than in leaves and in mature than in young leaves (). This pattern of expression agrees with our previous data that auxin from mature leaves, which is polarly transported through the stem, is decisive for rooting in carnation cuttings. Variations in the expression of this gene observed during cold storage (depending on the cultivar) might be related to the variation in PAT and rooting reported in previous studies.Citation12,Citation14,Citation15
Our current experiments suggest that pinching cuttings from mother plants reduces the expression of the gene DcAUX1. However the protein has a given turnover and it could still remain as a functional carrier for some time. The result might be an apparent “barrier effect”Citation17 which allows the progressive accumulation of IAA in the base of the cuttings, producing the peak necessary to induce a change of phase and starting the rooting process. According to proposed models,Citation18 IAA derepresses the ARF genes which activate the synthesis of transcription factors such as PLETHORA or SHORT ROOT/SCARECROW to organize a quiescent centre, which gives rise to the root apical meristem. Later, restoration of the PAT pathway guarantees the auxin flow necessary to maintain the growth of the new root.
Among our results, the variations observed in DcAUX1 expression might reflect the mutual interaction between PIN/AUX1 and PLETHORA genes.Citation19–Citation22 Low temperatures (storage in a cold chamber) presumably affected the frequency of the oscillation of gene expression, which finally would reach stable levels.
Figures and Tables
Figure 1 Variation in the expression of DcAUX1 in different organs of carnation cuttings during cold storage. The expression in a given organ after each storage period (3 days and 1, 3 and 5 weeks) is presented as a percentage on the sum of the values measured in all the organs analyzed after each four storage period. The scheme shows the different organs studied (I0, basal internode; I1, first internode; L1 and L2, mature leaves; L5, young leaves). The arrows represent the movement of auxin from leaves and the PAT pathway in the stem. The width of the arrows reflects the amount of auxin transported. Auxin from all the mature leaves is accumulated in the rooting zone of the cutting (I0). The data presented correspond to the carnation cultivar Solar.
Acknowledgements
This work was supported by the MEC/MICINN, Spain and FEDER, EU (grants AGL-2004-07902 and AGL-2008-02472) and a fellowship to M.R.O.-V.
Addendum to:
References
- Jarvis BC. Jackson MB. Endogenous control of adventitious rooting in non woody cuttings. New Root Formation in Plant and Cuttings 1986; Martinus-Nijhoff Kluwer Academic Publisher Group 191 - 222
- Gaspar T, Hofinger M. Davis TD, Haissig BE, Sankhla N. Auxin metabolism during root formation. Adventitious Root Formation in Cuttings. Advances in Plant Sciences Series 1988; 2:Dioscorides Press 117 - 131
- Haissig BE. Influence of indole-3-acetic acid on adventitious root primordia of brittle willow. Planta 1970; 95:27 - 35
- Eliasson L, Areblad K. Auxin effects on rooting in pea cuttings. Physiol Plant 1984; 61:293 - 297
- Katsumi M, Chiba Y, Fukuyama M. The roles of the cotyledons and auxin in the adventitious root formation of hypocotyl cuttings of light-grown cucumber seedlings. Physiol Plant 1969; 22:993 - 1000
- Liu JH, Reid DM. Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings IV. The role of changes in endogenous free and conjugated indole-3-acetic acid. Physiol Plant 1992; 86:285 - 292
- Parry G, Marchant A, May S, Swarup R, Swarup K, James N, et al. Quick on the uptake: characterization of a family of plant auxin influx carriers. J Plant Growth Regul 2001; 20:217 - 225
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, et al. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002; 14:589 - 597
- Morris DA, Friml J, Zazímalova E. Davies PJ. The transport of auxins. Plant Hormones. Biosynthesis, Signal Transduccion and Action 2004; Kluwer Academic Publishers 437 - 470
- Kramer EM, Bennett MJ. Auxin transport: a field in flux. Trends Plant Sci 2006; 11:382 - 386
- Garrido G, Cano EA, Arnao MB, Acosta M, Sánchez-Bravo J. Influence of cold storage period and auxin treatment on the subsequent rooting of carnation cuttings. Sci Hortic 1996; 65:73 - 84
- Garrido G, Cano EA, Acosta M, Sánchez-Bravo J. Formation and growth of roots in carnation cuttings: influence of cold storage period and auxin treatment. Sci Hortic 1998; 74:219 - 231
- Garrido G, Guerrero JR, Cano EA, Acosta M, Sánchez-Bravo J. Origin and basipetal transport of the IAA responsible for rooting of carnation cuttings. Physiol Plant 2002; 114:303 - 312
- Guerrero JR, Garrido G, Acosta M, Sánchez-Bravo J. Influence of 2,3,5-triiodobenzoic acid and 1-N-naphthylphthalamic acid on indoleacetic acid transport in carnation cuttings. Relationship with rooting. J Plant Growth Regul 1999; 18:183 - 190
- Garrido G, Arnao MB, Acosta M, Sánchez-Bravo J. Polar transport of indole-3-acetic acid in relation to rooting in carnation cuttings: influence of cold storage duration and cultivar. Biol Plant 2004; 47:481 - 485
- Oliveros-Valenzuela MR, Reyes D, Sánchez-Bravo J, Acosta M, Nicolás C. Isolation and characterization of a cDNA clone encoding an auxin influx carrier in carnation cuttings. Expression in different organs and cultivars and its relationship with cold storage. Plant Physiol Biochem 2008; 46:1071 - 1076
- Sánchez-Bravo J, Oliveros-Valenzuela R, Nicolás C, Acosta M. Growing in darkness. The etiolated lupin hypocotyls. Plant Signal Behav 2008; 3:406 - 408
- Benjamins R, Scheres B. Auxin: the looping star in plant development. Ann Rev Plant Biol 2008; 59:443 - 465
- Aida M, Beis D, Willemsen V, Blilou I, Galinha C, Nussaume L, et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004; 119:109 - 120
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Fryml J, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005; 433:39 - 44
- Grieneisen VA, Xu J, Maree AFM, Blilou I, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 2007; 449:1008 - 1013
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 2007; 449:1053 - 1057