Abstract
Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) produces a chlorosis-inducing phytotoxin coronatine (COR), which has multiple virulence functions in planta. One of the hallmarks of bacterial speck disease on tomato leaves is the formation of necrotic lesions surrounded by chlorosis. The physiological significance of COR-induced chlorosis in disease development is still unknown. In our recent publication in New Phytologist, we demonstrated that COR-induced effects on photosynthetic machinery resulted in the accumulation of reactive oxygen species (ROS). Tomato seedlings inoculated with Pst DC3000 and incubated in light showed more disease-associated necrotic cell death than inoculated seedlings incubated in either dark or low light conditions. Furthermore, COR suppressed the expression of thylakoid-localized Cu/Zn superoxide dismutase (Cu/Zn SOD), but not the cytosolic-localized Cu/Zn SOD. In this addendum, we propose a model for the function of COR as a regulator of plant ROS production in different cellular sites leading to disease-associated necrotic cell death during bacterial speck of tomato.
Several pathovars of Pseudomonas syringae produce the nonhost-specific phytotoxin coronatine (COR), a polyketide formed by the coupling of coronafacic acid (CFA) with coronamic acid (CMA) through an amide bond.Citation1 COR functions as a structural and functional analogue of endogenous plant signal molecules, including jasmonic acid (JA) and related signaling compounds such as methyl jasmonate (MeJA) and JA conjugated to isoleucine (JA-Ile).Citation2–Citation5 Plants utilize mutually antagonistic interactions of JA and salicylic acid (SA)-mediated defense signaling pathways to activate appropriate defense responses. COR is known to induce the JA pathway and function as suppressor of SA-mediated defense responses during pathogenesis.Citation6–Citation8 Studies using the biochemically-defined COR-defective mutant DB29 demonstrated that COR has unknown SA-independent functions during disease development.Citation7,Citation8
To investigate the SA-independent functions of COR in the pathogenicity of Pst DC3000, we took advantage of a tomato seedling assay that we previously establishedCitation9 and compared the expression profiles of tomato seedlings inoculated with Pst DC3000 and the COR-defective mutant DB29. The expression profiles showed that Pst DC3000 functions in a COR-dependent manner to reduce the expression of photosynthesis-related genes, including those involved in chlorophyll biosynthesis and the light and dark (Calvin-Benson cycle) reactions. Furthermore, the chlorophyll fluorescence measurements demonstrated that COR and Pst DC3000 induce a significant decrease in Fv/Fm at early time points, suggesting that COR from Pst DC3000 inhibits PSII before the onset of disease-associated necrotic cell death.Citation10
To investigate the role of the ROS elicited by COR on photosynthetic machinery, we first confirmed that ROS was produced in response to COR from Pst DC3000 with histochemical staining. Second, when seedlings were pre-treated with ROS inhibitors, 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) and diphenylene iodonium (DPI) and then inoculated with Pst DC3000, disease-associated necrotic cell death was significantly reduced compared to control inoculation with Pst DC3000. Third, seedlings inoculated with Pst DC3000 and incubated in light showed more disease-associated necrotic cell death than in either dark or low light conditions. Taken together, these findings suggested that COR-induced ROS contributes to the disease-associated necrotic cell death in tomato.Citation10
ROS functions in the stimulation of hypersensitive cell deathCitation11,Citation12 and disease-associated necrotic cell death.Citation13,Citation14 The signals associated with plant innate immunity include ion flux, the oxidative burst, activation of MAP kinase cascades, and defense gene expression; these responses are triggered by the perception of pathogen-associated molecular patterns (PAMPs).Citation15 Thus, during the early stages of infection, Pst DC3000 might use several effectors or virulence factors to suppress PAMP-inducible ROS and the resulting PAMP-triggered immunity (PTI).Citation16,Citation17 However, the precise mechanism and the suppressors of PTI-associated ROS are unknown. Our results indicate that COR induces ROS production in the later stages of infection (24 hpi). Thus, we investigated whether COR could regulate ROS production in different cellular sites and different infection stages by analyzing expression profiles of cytosolic and thylakoid Cu/Zn superoxide dismutases. The expression of cytosolic Cu/Zn superoxide dismutase (Cu/Zn cSOD) was induced by treatment with COR or by inoculation with Pst DC3000, but not DB29, suggesting that COR-inducible ROS-detoxifying enzymes might protect Pst DC3000 from PTI during the early stages of interaction. We further tested if the COR− mutant DB29 could multiply to higher levels when the PTI-mediated ROS was eliminated. In support of this hypothesis, the COR− mutant DB29 multiplied to higher levels in seedlings pre-treated with DPI (). It is important to note that the expression of the thylakoid-localized Cu/Zn SOD (Cu/Zn tSOD) was suppressed by COR or Pst DC3000, but not by DB29, suggesting that the reduced expression of ROS-detoxifying enzyme(s) might be related to higher levels of ROS in the chloroplast. Taken together, our findings suggest that COR may suppress the primary PTI by decreasing cytolsolic ROS and may also function to regulate ROS production at later stages of disease development to induce disease-associated necrotic cell death.
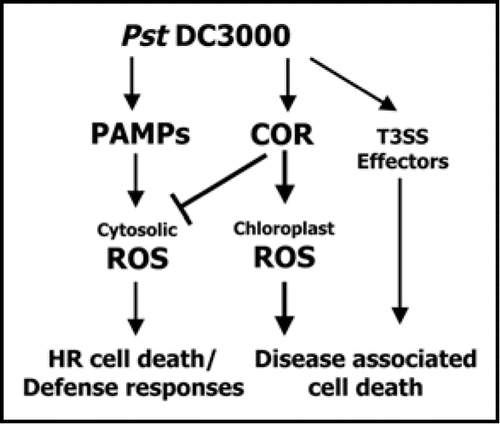
In conclusion, our study has revealed a role for COR-induced ROS in modulating disease-associated necrotic cell death during bacterial speck disease in tomato. As summarized in , the COR produced by Pst DC3000 induces ROS by affecting photosynthetic machinery and ROS-detoxifying enzymes, and then contributes to disease-associated necrotic cell death. However, the application of purified COR onto leaves elicits chlorosis that is not associated with necrotic cell death.Citation1,Citation5 Furthermore, COR and selected effector proteins have been reported to function through COI-1 dependent pathways to promote virulence.Citation18–Citation20 Therefore, it is not clear whether COR-induced ROS-mediated necrotic cell death is mechanistically related to effector-mediated cell death during the Pst DC3000 infection process. Thus, the identification of host molecules regulated by COR and necrosis-inducing effector proteins is necessary to understand disease-associated necrotic cell death during bacterial speck disease of tomato. It remains possible that COR and necrosis-inducing effector proteins might target host signaling components to regulate disease-associated necrotic cell death during bacterial speck disease development of tomato.
Figures and Tables
Figure 1 Bacterial growth of P. syringae pv. tomato DC3000 (Pst DC3000) and the COR defective-mutant DB29 in tomato seedlings leaves pretreated with diphenylene inodonium (DPI), which inhibits the activity of membrane-bound NADPH oxidase. Seedlings were inoculated with Pst DC3000 or DB29 following pretreatment with water (control) or DPI (20 mM) for 24 h. The population of Pst DC3000 were measured at 0 days post-inoculation (dpi) and 4 dpi. Vertical bars indicate the standard error for three independent experiments.
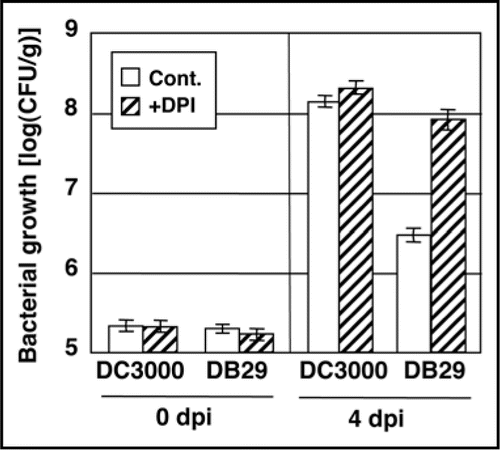
Figure 2 A model for the virulence function of COR in the pathogenicity of P. syringae pv. tomato DC3000 (Pst DC3000). COR from Pst DC3000 regulates both cytosolic and chloroplast ROS homeostasis, and COR-inducible ROS might function with T3SS effector proteins to induce disease-associated cell death.
Acknowledgements
C. Bender acknowledges support from National Science Foundation grant IBN-0620469 and the Oklahoma Agricultural Experiment Station. Y. Ishiga was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science (JSPS). The OSU Microarray Core Facility was supported by grants from NSF (EOS-0132534) and NIH (1P20RR16478-02 and 5P20RR15564-03).
Addendum to:
References
- Bender CL, Alarcon-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 1999; 63:266 - 292
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994; 6:751 - 759
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004; 16:2117 - 2127
- Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Neisel U, Bublitz F. The Pseudomonas phytotoxin coronatine mimics octadecanoid signaling molecules of higher plants. FEBS Lett 1994; 345:9 - 13
- Uppalapati SR, Patricia A, Weng H, Palmer DA, Mitchell RE, Jones W, et al. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J 2005; 42:201 - 217
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 2002; 5:325 - 331
- Brooks DM, Bender CL, Kunkel BN. The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defense in Arabidopsis thaliana. Mol Plant Pathol 2005; 6:629 - 639
- Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, et al. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol Plant-Microbe Interact 2007; 20:955 - 965
- Uppalapati SR, Ishiga Y, Wangdi T, Urbanczyk-Wochniak E, Ishiga T, Mysore KS, et al. Pathogenicity of Pseudomonas syringae pv. tomato on tomato seedlings: phenotypic and gene expression analyses of the virulence function of coronatine. Mol Plant-Microbe Interact 2008; 21:383 - 395
- Ishiga Y, Uppalapati SR, Ishiga T, Elavarthi S, Martin B, Bender CL. The phytotoxin coronatine induces light-dependent reactive oxygen species in tomato seedlings. New Phytol 2009; 181:147 - 160
- Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 2002; 99:517 - 522
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 2004; 55:373 - 399
- Venisse JS, Gullner G, Brisset MN. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora. Plant Physiol 2001; 125:2164 - 2172
- Coego A, Ramirez V, Ellul P, Mayda E, Vera P. The H2O2-regulated Ep5C gene encodes a peroxidase required for bacterial speck susceptibility in tomato. Plant J 2005; 42:283 - 293
- Schwessinger B, Zipfel C. News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 2008; 11:389 - 395
- Espinosa A, Alfano JR. Disabling surveillance: bacterial type III secretion system effectors that suppress innate immunity. Cell Microbiol 2004; 6:1027 - 1140
- Nomura K, Melotto M, He SY. Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr Opin Plant Biol 2005; 8:361 - 368
- Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J 2003; 36:485 - 499
- He P, Chintamanani S, Chen Z, Zhu L, Kunkel BN, Alfano JR, et al. Activation of a COI1-dependent pathway in Arabidopsis by Pseudomonas syringae type III effectors and coronatine. Plant J 2004; 37:589 - 602
- Thilmony R, Underwood W, He SY. Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J 2006; 46:34 - 53