Abstract
In well aerated soils, iron exists, mainly as scarcely soluble oxides and oxi-hydroxides and, therefore, not freely available to plants uptake, notwithstanding its abundance. Multifaceted strategies involving reductase activities, proton processes, specialized storage proteins, and other, act in concert to mobilize iron from the environment, to take it up and to distribute it inside the plant. Because of its fundamental role in plant productivity several questions concerning homeostasis of iron in plants are currently a matter of intense debate. We discuss some recent studies on Strategy I responses in dicotyledonous plants focusing on metabolic change induced by iron deficiency, mainly concerning the involvement of mitochondria.
Introduction
Iron deficiency is a widespread agricultural problem in many crops grown in alkaline, calcareous soils in which iron, although abundant, is often not soluble and therefore unavailable for root uptake.Citation1 The importance of iron for plants is due to the existence of two stable, but convertible forms, ferric [Fe(III)] and ferrous [Fe(II)]. As a component of many vital proteins, including cytochromes and Fe-S cluster proteins of the electron transport chains, it is required for a wide range of biological functions.Citation2 In fact, it takes part in fundamental processes, mainly in the oxidative (respiratory) and biosynthetic (photosynthetic) pathways.Citation3,Citation4
Iron uptake and homeostasis are tightly regulated in plants to ensure both a sufficient supply from the soil and the avoidance of a toxic excess in the cell. Iron deficiency induces various responses at the root level, aimed to increase the availability of the ion in the rhizosphere. Strategy I plants (dicotyledonous and non-graminaceous plants) are able to respond to a lack of iron in the soil by increasing, (i) the iron reduction activity of root tissues, (ii) the acidification of the rhizosphere to increase iron solubility and (iii) the uptake activities in rhizodermal root cells (i.e., Fe(III)-chelate reductase [FC-R], H+-ATPase and iron regulated transporters [IRT], respectively).Citation3
General Metabolic Responses
Several changes occur at the metabolic level in order to sustain the necessity to increase Fe uptake in Fe-deficient plants.Citation5 These changes include, (i) increase in the activity of PEPC and of several enzymes of the glycolytic pathway such as glyceraldehyde 3-phosphate dehydrogenase, pyruvate kinase and phosphofructo kinase 1 and of the Krebs cycle such as citrate synthase, isocitrate dehydrogenase, fumarase and aconitase;Citation5 (ii) shift in the redox state of the cytosol.Citation2,Citation6 Some of these increased activities are associated with an enhanced expression of the corresponding genes.Citation7 Organic acid synthesis and CO2 dark fixation is enhanced under Fe-starvation—during which phosphoenol pyruvate carboxylase (PEPC) activity has been shown to increase several fold.Citation6,Citation8,Citation9
The anaplerotic role of PEPC has been characterized in roots of cucumber grown under Fe deficiencyCitation9 and in other Strategy I species.Citation6,Citation10 When grown in iron-limiting conditions, Strategy I plants accumulate organic acids—mainly citrate and malate—in rootsCitation11,Citation12 and leavesCitation13,Citation14 It is widely assumed that citrate plays an important role in the transport of iron in rootsCitation15–Citation18 and in its translocation, via xylem, to mesophyll cells.Citation11,Citation17,Citation19
Under Fe deficiency both the FC-R and H+-ATPase activities are greatly enhanced, leading to a strong request of energy in the form of NAD(P)H and ATP. It has been found that several free cytosolic dehydrogenases (malic dehydrogenase, malic enzyme, isocitrate dehydrogenase) and dehydrogenases from the glycolytic and pentose phosphate pathways were enhanced under Fe deficiency.Citation8,Citation20 However other processes that require energy are increased. For instance, the increase in the mRNA production and successively the synthesis of the corresponding enzymes. It has been shown that both RNA and protein synthesis were increased under Fe deficiency.Citation21 Usually cells carry out, at faster rate, glycolysis and mitochondrial oxidative processes to sustain the increased request of energy and metabolites. In fact, the rate of oxygen consumption in apical root segments was shown to be increased in this condition suggesting an enhanced respiratory activity by mitochondria.Citation6,Citation20,Citation22 However, it has been shown that O2 can be used by FC-R itself when plants are grown in the complete absence of iron instead of mitochondria.Citation6,Citation22 Unfortunately, this reaction could generate H2O2, O2−or other ROS. On the other hand, some of the enzymes involved in the detoxification of these compounds were found to be overexpressed in cucumberCitation23 and in sugar beet roots,Citation24 in iron-limiting conditions.
Mitochondrial Responses
The enzymatic complexes of the mitochondrial electron transport chain (mtETC) contain several Fe-S cluster and heme groups. In the absence of iron, heme and Fe-S cluster synthesis should be impaired and indeed the de novo synthesis of iron-containing proteins occurs at a lower rate in mitochondria of iron deficient plants.Citation5 In fact, under Fe deficiency, it has been shown that the level of some iron containing components (cytochromes and Fe-S clusters) were greatly diminished in mitochondria of Fe-deficient sycamore cellsCitation25 and cucumber roots.Citation22 Moreover, the morphology and the ultrastructure of the mitochondria are affected by Fe deficiency. Mitochondria from Fe-deficient sycamore cells displayed a more dilute matrix with less pronounced cristae consistent with the marked decline in cytochromes and Fe-S clusters.Citation25 Interestingly, in Fe-deficient cucumber root, mitochondria displayed a particular handlebar-like structure and they seem to aggregate each others.Citation22 Generally, the morphology of mitochondria is very dynamic, often changing shape within a cell and from one cell type to another. Mitochondria are also dynamic, in terms of their movement within cells, moving rapidly along cellular structures, such as actin and microtubule cytoskeleton. The main mitochondrial processes that regulate the shape and the movement of the organelles in the cell are the fusion and the fission. These two processes compensate each other leading to the maintenance of a relatively constant number of mitochondria in the cells. When mitochondrial fusion predominates, mitochondria become highly interconnected or networked. On the contrary, fragmentation of mitochondria and inhibition of fusion accompany the earliest steps of mitochondria-mediated apoptosis. Several authors suggested that the number of mitochondria were enhanced in Fe-deficient cells,Citation25–Citation28 so we might hypothesize that this nutritional stress could bring to a modification of the mitochondrial fusion/fission ratio. Moreover, a similar handlebar-like structure observed in Fe-deficient cucumber root has been observed in Arabidopsis DRP3A mutant.Citation29 Until now there is not specific evidence about a possible impairment of fusion and fission processes under Fe deficiency, but the changes in the number,Citation25–Citation28 in the shapeCitation22 and in the ultrastructuresCitation25 of mitochondria suggest that a low availability of iron, by altering de novo synthesis of Fe-S clusters and heme containing proteins, impairs the mitochondrial dynamic processes.
Energetic Cell Adaptation
As stated before the lack of Fe affects the synthesis of Fe-heme and Fe-S cluster impairing mitochondrial efficiency.Citation5,Citation22 Thus, under Fe starvation, the cell reach a kind of energetic emergency status in which, from one hand, the cell needs more energy for the activation of the Strategy I responses and from the other hand, the primary source of energy (mitochondrion) is impaired.
What the cell can do? To survive under Fe-limiting growing condition the cell must find alternative energy producing mechanisms. Glycolysis is the first aid for the cell to avoid the cellular energetic collapse.
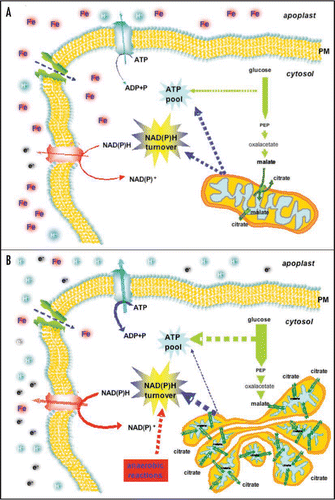
The activation of anaerobic pathways might be related either to a drop in the O2 concentration or to the inefficiency of mitochondria. As occurs in the muscle of mammals in which a strong energetic effort, caused by an enhanced muscular activity, activate the anaerobic pathway to produce energy instead of insufficient respiratory activity. The root cells of Fe-deficient plant could undergo a similar scenario: the lack of Fe induces a strong energetic request for its acquisition and at the same time impairs the mitochondrial respiratory chain. Fe deficiency could simulate a kind of “cellular effort” in which to overcome a respiratory chain impairment, the cell needs alternative pathways to sustain both energetic requirements and NAD(P)H turnover. The cell could then enable (a) glycolytic and anaerobic pathways to run faster, to sustain ATP synthesis, and (b) organic acid exchange between mitochondria and cytosol to sustain NAD(P)H turnover and carbon flux.Citation22
Which Could be the Fate of Plant Mitochondria Under Fe-Deficiency?
Apparently, Fe-deficient mitochondria seem to become the biggest ‘victim’ of the Fe starvation in root tissues, since they required at least 40 Fe atoms/respiratory unit to work. This organelles are strongly affected by this nutritional disorder both at the functional and the morphological levels.Citation22,Citation25 Moreover, the low Fe content inside the mitochondria, observed in those studies, also induces a decrease in the content of mitochondrial ferritin (Vigani G and Zocchi G, unpublished). Ferritin is a Fe-storage protein mainly located in the plastids.Citation30,Citation31 Recently, it has been observed the presence of this protein also in the plant mitochondria, having the important role of Fe sequestration to regulate iron homeostasis and to prevent the formation of reactive oxygen species and the subsequent oxidative damages.Citation32,Citation33 On the other hand, plant mitochondria possess an electron transport chain where superoxide anion may be generated by univalent reactions at the level of complex I or III.Citation34 For this reason, mitochondria are well equipped with enzymes to prevent and scavenge ROS formation.Citation35,Citation36 Sequestration of potential harmful ferrous ions has been suggested as a possible mechanism to overcome this problem.Citation32 Therefore, the sequestration of iron by ferritin in chloroplasts and mitochondria, two of the major sites of ROS generation in plant cells,Citation35,Citation37 can constitute an additional strategy to prevent this oxidative damage.Citation32 However, it seems rationale that the low level of iron inside the mitochondria induces a repression of ferritin synthesis, avoiding a further depletion of the iron concentration by the ferritin itself, since iron is essential for the correct assembly of the respiratory units.
Fe deficiency leads to an almost Fe-free mitochondria in the plant tissues leading to a decrease in the respiratory chain efficiency. A question rises: is the mitochondrion destined to loss, in this condition, its peculiar functions in the cell? Recently, it has been observed an overexpression of the mitochondrial di-tricarboxylic acid carrier (DTC) under Fe limiting conditions suggesting an increased communication between the cytosolic and the mitochondrial pools of organic acids.Citation22 Organic acids are important for the translocation of Fe throughout the plant, as supply of carbon skeleton and for the regulation of cytosolic pH.Citation6,Citation18 The increased organic acid flux between cytosol and mitochondria could be a strategy of the cell to allow a faster turnover of reducing equivalents.Citation22,Citation38 Since both the cytosolic and the mitochondrial NAD(P)H need to be re-oxidized to keep the catabolic pathways working, a less efficient respiratory chain activity could impair this turnover, leading to a higher ratio of reduced over oxidized pyridine nucleotide compounds, as showed in bean for NADPH/NADP+ ratio.Citation39 A lower turnover rate might in turn decrease the rate of other metabolic pathways (for instance glycolysis) causing an imbalance of energy and metabolite production. However, in Plantago lanceolata the total pool of pyridine nucleotide was more oxidized under Fe deficiency, although the NADPH/NADP+ pool was slightly higher.Citation40 On the contrary, the NAD(P)H/NAD(P)+ ratio was lower in Fe-deficient sugar beet and tomato roots with respect to the control.Citation6,Citation18 These data have been explained as the major request of reducing equivalent by the induction of the FC-R activity. Collectively these data show how complex is the balance between the reducing and oxidising processes and that Fe deficiency strongly induces the NAD(P)H oxidising activities over the NAD(P)+ reducing activities.
Since the major site of the cell capable to oxidise NAD(P)H, is the respiratory chain, under Fe starvation which are the reactions able to oxidize all the reducing equivalent produced by the glycolysis and TCA? One may hypothesize that the increased request of reducing equivalents due to the induction of FC-R activity in Fe-deficient roots could be the sole responsible of the decrease in the NAD(P)H/NAD(P)+ ratio. However, even this is true, it seems unlike that FC-R alone may be responsible for the change in this ratio. At this point it would be rationale for an aerobic tissue to change to anaerobic pathways, as reported by López-Millán et al.Citation6,Citation18 that showed an increased activities in the fermentation enzymes such as lactate dehydrogenase (LDH) and of pyruvate decarboxylase (PDC) and of several other cytosolic NADH-consuming enzymes, such as isocitrate dehydrogenase (ICDH) and malate dehydrogenase (MDH) that could contribute to the oxidation of the pyridine nucleotide pool in tomato and sugar beet roots. These data are also confirmed by microarray analysis on Fe-deficient Arabidopsis.Citation7 This hypothesis will completely exclude the mitochondria from being able to carry out any oxidative process of reduced pyridine nucleotides. We suggest that also the mitochondria might participate in the oxidation of NAD(P) H under Fe deficiency (). In fact, plant mitochondria are characterized by the presence of several alternative pathways which are able to perform oxidation of reducing equivalent such as alternative NAD(P)H dehydrogenases (NAD(P)H DHs), alternative oxidase (AOX) and metabolite shuttles. As reported by Vigani et al.Citation22 NAD(P)H DHs activities seem to be activated to bypass the strong loss of Complex I and Complex II activities occurring in Fe-deficient cucumber roots. The external and internal localization of these enzymes in the inner mitochondrial membrane allow them to oxidize the NAD(P)H both from the cytosol and from the matrix, but until now there is no evidence supporting their involvement under Fe deficiency. Concerning the AOX activity, in the literature there are only indirect data relative to the oxygraph analysis in which the SHAM-sensitive pathway decrease in Fe-deficient root tissues with respect to the control.Citation6,Citation18,Citation22
Moreover, the cytosolic reducing equivalents may be transferred to mitochondria through several metabolite shuttles that operate between the two compartments. The malate/oxaloacetate,Citation41 and the malate/aspartate shuttles,Citation42 are the most extensively studied redox exchange mechanisms in plant cells. In yeast and animal systems, a mitochondrial glycerol-3-phosphate (G-3-P) shuttle has also been described.Citation43–Citation45 Recently in Arabidopsis a mitochondrial G-3-P shuttle has been found.Citation46,Citation47 According to the model described for yeast and animal cells,Citation48 a mitochondrial G-3-P shuttle involves the combined actions of a cytosolic NAD-dependent G-3-P dehydrogenase (GPDH) and a mitochondrial flavin adenine dinucleotide (FAD) dependent G-3-P dehydrogenase:ubiquinone oxidoreductase (FAD-GPDH). So far, no evidence has been found about the involvement of the latter metabolite shuttle in Fe-deficient plants, but in Fe-deficient roots of Arabidopsis an induction of the transcript of the genes encoding for G-3-P shuttle was observed (Vigani G, et al. unpublished).
Conclusion
Despite mitochondria are an important target of the Fe deficiency, they still play a pivotal role in the metabolic changes occurring in this condition. The presence of several alternative oxidative pathways allows them to still regulate the energetic demand of the cell in this condition by keeping a high turnover of pyridine nucleotide and avoiding ROS formation. In fact, the alternative NAD(P)H DHs allow both, (a) to carry forward the electron transport along the respiratory chain bypassing the decreased activities of complex I and II; and (b) to maintain a high NAD(P)H turnover in the cell. The activation of the metabolite shuttles (i.e., DTC and G-3-P) allows both, (a) the transfer of reducing equivalent between cytosolic and mitochondrial compartments and (b) the exchange of carbon skeleton.
The particular aggregation and the handlebar-like structure observed in mitochondria of Fe-deficient cucumber root could be interpreted as a strategy of the cell to overcome the energetic emergency status by approaching these organelles each other thus enhancing the mitochondrial communication. We suggest that, by enhancing the number of mitochondria, the cell could increase the number of compartments that can sustain the turnover of reducing equivalents. Therefore, mitochondria from Fe-deficient roots are limited in the electron transport chain and ATP synthesis but they could maintain the capacity to reduce the NAD(P)H elevated ().
Abbreviations
| mtETC | = | mitochondrial electron transport chain |
| DTC | = | di-tricarboxylic acid carrier |
| TCA cycle | = | tricarboxylic acid cycle |
Figures and Tables
Figure 1 Model of the Fe deficiency effects on the metabolism of cucumber root cells. In the control condition (A) mitochondria are able to satisfy the energetic and the NAD(P)H turnover requests of the cell. In Fe deficiency (-Fe) condition (B), the ATP and NAD(P)H request are enhanced and the respiratory chain is strongly impaired leading the cellular metabolism to change several pathways. To overcome the energetic emergency status, the cell increases (i) glycolysis; (ii) the synthesis of DTC protein leading to an enhanced of citrate/malate exchange between mitochondrial matrix and cytosol; (iii) the anaerobic metabolism. Changes in the arrow thickness indicates the changes in the rate of metabolic processes occurring under Fe deficiency condition. Abbreviations: FC-R, Ferric chelate-reductase; IRT, iron regulated transporter; DTC, di-tricarboxylic acid carrier; PM, plasma membrane.
Acknowledgements
The research was financially supported by grant from MIUR.
References
- Lindsay WL, Schwab AP. The chemistry of iron in soils and its availability to plants. J Plant Nutr 1982; 5:821 - 840
- Schmidt W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol 1999; 141:1 - 26
- Curie C, Briat JF. Iron transport and signalling in plants. Annu Rev Plant Biol 2003; 54:183 - 206
- Hell R, Stephan UW. Iron uptake, trafficking and homeostasis in plants. Planta 2003; 216:541 - 551
- Zocchi G. Barton LL, Abadèa J. Metabolic changes in iron-stressed dicotyledonous plants. Iron Nutrition in Plants and Rhizospheric Microorganisms 2006; Dordrecht, The Netherlands Springer 359 - 370
- López-Millán AF, Morales F, Andaluz S, Gogorcena Y, Abadía A, De Las Rivas J, et al. Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant physiol 2000; 124:885 - 897
- Thimm O, Essigmann B, Kloska S, Altmann T, Buckhout TJ. Response of arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol 2001; 127:1030 - 1043
- Rabotti G, De Nisi P, Zocchi G. Metabolic implications in the biochemical responses to iron deficiency in cucumber (Cucucmis sativus L.) roots. Plant Physiol 1995; 107:1195 - 1199
- De Nisi P, Zocchi G. Phosphoenolpyruvate carboxylase in cucumber (Cucumis sativus L.) roots under iron deficiency: activity and kinetic characterisation. J Exp Bot 2000; 352:1903 - 1909
- Ollat N, Laborde B, Neveux M, Diakou-Verdin P, Renaud C, Moing A. Organic acid metabolism in root of various grapevine (Vitis) rootstocks submitted to iron deficiency and bicarbonate. J Plant Nutr 2003; 26:2165 - 2176
- Brown JC. Iron and Ca uptake as related to root-sap and stem-exudate citrate in soybeans. Physiol Plant 1966; 19:968 - 976
- Alhendawi RA, Römheld V, Kirby EA, Marschner H. Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum and maize. J Plant Nutr 1997; 20:1731 - 1753
- Iljin WS. Metabolism of plants affected with lime induced chlorosis: II. Organic acids and carbohydrates. Plant and Soil 1951; 3:339 - 351
- Landsberg EC. Organic acid synthesis and release of hydrogen ions in response to iron deficiency stress of mono and dicotyledonous plant species. J Plant Nutr 1981; 3:579 - 591
- Tiffin LO. Iron translocation: II Citrate/iron ratios in plant stem exudates. Plant Physiol 1966; 41:515 - 518
- White MC, Baker FD, Chaney RL, Decker AM. Metal complexation in xylem fluid: II. Theoretical equilibrium model and computational computer program. Plant Physiol 1981; 67:301 - 310
- Abadía J, López-Millán AF, Rombolà A, Abadía A. Organic acid and Fe deficiency: a review. Plant Soil 2002; 241:75 - 86
- López-Millán AF, Morales F, Gogorcena Y, Abadia A, Abadia J. Metabolic responces in iron deficient tomato plants. J Plant Physiol 2009; 166:375 - 384
- Brown JC, Chaney RL, Ambler JE. A new tomato mutant inefficient in the transport of iron. Physiol Plant 1971; 25:48 - 53
- Espen L, Dell'Orto M, De Nisi P, Zocchi G. Metabolic responses in cucumber (Cucumis sativus L.) roots under Fe-deficiency: a 31P-nuclear magnetic resonance in vivo study. Planta 2000; 210:985 - 992
- Pontiggia A, De Nisi P, Zocchi G. Effect of iron deficiency on RNA and protein synthesis in cucumber roots. J Plant Nutr 2003; 10:2177 - 2186
- Vigani G, Maffi D, Zocchi G. Iron availability affects the function of mitochondria in cucumber root. New Phytol 2009; 182:127 - 136
- Rabotti G, Zocchi G. Plasma membrane-bound H+-ATPase and reductase activities in Fe-deficient cucumber roots. Physiol Plant 1994; 90:779 - 785
- Zaharieva TB, Abadía J. Iron deficiency anhances the level of ascorbate, glutathione and related enzymes in sugar beet roots. Protoplasma 2003; 221:269 - 275
- Pascal N, Douce R. Effect of iron deficiency on the respiration of sycamore (Acer pseudoplatanus L.) calls. Plant Physiol 1993; 103:1329 - 1338
- Landsberg EC. Function of rizhodermal transfer cells in the Fe stress response mechanism of Capsicum annuum L. Plant Physiology 1986; 82:511 - 517
- Landsberg EC. Transfer cell formation in sugar beet roots induced by latent Fe deficiency. Plant & Soil 1994; 165:197 - 205
- Dell'Orto M, Pirovano L, Villalba JM, Gonzalez-Reyes JA, Zocchi G. Localization of the plasma membrane H+-ATPase in Fe-deficient cucumber roots by immunodetection. Plant & Soil 2002; 241:11 - 17
- Logan DC, Scott I, Tobin AK. ADL2a, like ADL2b, is involved in the control of higher plant mitochondrial morphology. J Exp Bot 2004; 55:783 - 785
- Seckbach S. Ferreting out the secrets of plant ferritin. J Plant Nutr 1982; 5:369 - 394
- Proudhon D, Wei J, Briat JF, Theil EC. Ferritingene organization: differences between plants and animals suggest possible kingdom-specific selective constraints. J Mol Evol 1996; 42:325 - 336
- Zancani M, Peresson C, Biroccio A, Federici G, Urbani A, Murgia I, et al. Evidence for the presence of ferritin in plant mitochondria. Eur J Biochem 2004; 271:3657 - 3664
- Ravet K, Touraine B, Boucherez J, Briat JF, Gaymard F, Cellier F. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 2009; 57:400 - 412
- Braidot E, Petrussa E, Vianello A, Macrì F. Hydrogen peroxide generation by higher plant mitochondria oxidizing complex I or complex II substrates. FEBS Lett 1999; 451:347 - 350
- Møller IM. Plant mitochondria and oxidative stress: electron transport, NADPH turnover and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:561 - 591
- Casolo V, Braidot E, Chiandussi E, Macrì F, Vianello A. The role of mild uncoupling and non-coupled respiration in the regulation of hydrogen peroxide generation by plant mitochondria. FEBS Lett 2000; 474:53 - 57
- Alscher RG, Donahue JN, Cramer CL. Reactive oxygen species and antioxidants: relationship in green cells. Physiol Plant 1997; 100:224 - 233
- Bienfait HF. Is there a metabolic link between H− excretion and ferric reduction by roots of Fe-deficient plant? A viewpoint. J Plant Nutr 1996; 19:105 - 129
- Sijmons PC, Van Den Briel W, Bienfait HF. Cytosolic NADPH is the electron donor for extracellular FeIII reduction in iron-deficient bean roots. Plant Physiol 1984; 75:219 - 222
- Schmidt W, Schuck C. Pyridine nucleotide pool size changes in iron-deficient Plantago lanceolata roots during reduction of external oxidants. Physiol Plant 1996; 98:215 - 221
- Ebbighausen H, Chen J, Heldt HW. Oxaloacetate translocator in plant mitochondria. Biochim Biophys Acta 1985; 810:184 - 199
- Journet EP, Neuburger M, Douce R. The role of glutamate oxaloacetate transaminase and malate dehydrogenase in the regeneration of NAD+ for glycine oxidation by spinach leaf mitochondria. Plant Physiol 1981; 67:467 - 469
- Ansell R, Granath K, Hohmann S, Thevelein JM, Adler L. The two isoenzyme for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J 1997; 16:2179 - 2187
- Larsson C, Pahlman IL, Ansell R, Rigoulet M, Adler L, Gustafsson L. The importance of the glycerol 3-phosphate shuttle during aerobic growth of Saccharomyces cerevisiae. Yeast 1998; 14:347 - 357
- Rigoulet M, Aguilaniu H, Averet N, Bunoust O, Camougrand N, Grandier-Vazeille X, et al. Organization and regulation of the cytosolic NADH metabolism in the yeast Saccharomyces cerevisiae. Mol Cell Biochem 2004; 256:73 - 81
- Shen W, Wei Y, Dauk M, Zheng Z, Zou J. Identification of a mitochondrial glycerol-3 phosphate dehydrogenase from Arabidopsis thaliana: evidence for a mitochondrial glycerol-3 phosphate shuttle in plants. FEBS Lett 2003; 536:92 - 96
- Shen W, Wei Y, Dauk M, Tan Y, Taylor DC, Selvaraj G, et al. Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD1 ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis. Plant Cell 2006; 18:422 - 441
- Lehninger AL, Nelson AD, Cox MM. Lehninger AL, Nelson AD, Cox MM. Oxidative phosphorylation and photophosphorylation. Principles of Biochemistry 1993; 2nd New York Worth Publishers 585 - 586