Abstract
Transmission of plant viruses is the result of interactions between a given virus, the host plant, and the vector. Most research has focused on molecular and cellular virus-vector interactions, and the host has only been regarded as a reservoir from which the virus is acquired by the vector more or less accidentally. However, a growing body of evidence suggests that the host can play a crucial role in transmission. Indeed, at least one virus, Cauliflower mosaic virus, exploits the host's cellular pathways to form specialized intracellular structures that optimize virus uptake by the vector and hence transmission.
Transmission is a step in a virus's life cycle that is often neglected. Nonetheless, it is obvious that also this step is obligatory for a virus, as it could not maintain itself without dispersing to other hosts and infecting them. Most plant viruses are transmitted by insects, using two different strategies: “circulant transmission” where the virus, once taken up by the vector during feeding on an infected plant, passes from the intestine via the body lumen to the salivary glands and is finally inoculated with the saliva into a new host plant; the second strategy is “non-circulant transmission” where transmissible virus particles attach only to the exterior mouthpieces of the insect from which they are released into a new host. Whereas the first strategy obviously requires highly specific interactions between the virus and the vector to allow for passage of the virus through the vector, non-circulant transmission was initially thought of as a more or less accidental event, where virus sticks non-specifically to the mouthpieces. However, it becomes more and more evident that also non-circulant transmission is the result of sophisticated interactions between a given virus, a host and a vector. The vectors are most often aphids that, due to their non-destructive feeding behavior, are ideally suited as virus vectors. In fact, once landed on a plant, aphids first probe the prospective food source by short, only seconds lasting intracellular punctures in epidermis and mesophyll cells that do not even kill the punctured cells.Citation1 After these exploratory punctures and when they judge the plant as suited, the aphids insert their proboscis-like mouthpieces (stylets) into the phloem and feed from its sap for time spans that may exceed several hours. Depending on the tissues they infect, plant viruses can be acquired by aphids during either or only one of the two puncture phases. For example, Luteoviruses are only acquired from the vascular tissues,Citation2 whereas Cauliflower mosaic virus is acquired from both tissues.Citation3
Cauliflower mosaic virus (CaMV) is one of the best studied viruses on what concerns non-circulant transmission, the most often used transmission mode employed by plant viruses. For its transmission, a transmissible complex must form that attaches to a protein receptor located in the stylets of the aphid.Citation4 This complex is not only, as for some viruses, composed of the virus particle, but also, as for many non-circulantly transmitted plant viruses, of a viral helper protein that with one domain interacts with the virus particle and with another with the stylet receptorCitation5 (). The helper protein of CaMV, P2, seems to have no other function but to assist in transmission as CaMV mutants deleted of P2 are perfectly infectious but not transmissible.Citation6 A puzzling fact is that P2 may be acquired independently of the virus particle, meaning that it alone can bind to the stylet receptor and that virus particles either attach concomitantly with P2 onto the stylets or later attach to pre-bound P2. This has consequences for the composition of the transmitted viral population as it can be compiled of virus particles originating from the same cell from which P2 was acquired, but also from other cells and even sieve tubes that themselves do not contain P2.Citation3 In fact, this potentially sequential acquisition mode of CaMV by the vector is controlled by the intracellularCitation7 and tissue-specific localization of P2 that is only found in epidermis and parenchyma cells.Citation3 In these cells, P2 localizes exclusively in a single viral inclusion, the transmission body, that has been proposed and recently been shown to be specialized for transmission:Citation8–Citation10 if this structure does not form, CaMV can not be taken up by the aphid, even if functional P2 is present in the infected cell.
This posed the interesting question how the transmission body forms during infection because elucidating this mechanism would show that CaMV hijacks cellular pathways for the sole purpose to ensure its transmission. It was known that besides the single transmission body a second type of viral inclusion bodies is found in infected cells: the numerous “electron-dense inclusions” that are assumed to be the virus factories () where all viral synthesis occursCitation11 and where most virus particles accumulate. However, P2 was never described in the factories, presenting the paradox: if it is translated in the factories why is it not found there? Of different possible scenarios we chose to test the hypothesis that P2 is produced in the factories and then exported. Protoplasts were transfected with CaMV particles and kinetics of P2 accumulation followed by immunofluorescence. The results showed that P2 is indeed translated in the viral factories but then associates temporally with microtubules before finally condensing into a single transmission body. Also the other known components of the transmission body, the viral protein P3 and to a lesser degree, some virus particles, followed the same route from viral factories to the transmission body.
Experiments with cytoskeleton drugs confirmed that transient localization of transmission body components with microtubules, but not with actin filaments, is necessary for transmission body formation. The results also indicated that both microtubules and actin filaments are apparently not required for other steps of the intracellular infection cycle because formation of viral factories was only slightly inhibited by the drugs.
The results show that CaMV specifically uses the microtubule cytoskeleton to form the transmission body and thus enable vector transmission. Consequently, non-circulant transmission of at least this virus is not a random event where the vector takes up some transmissible complexes by chance. It is rather the result of highly specific interactions, where the virus “intentionally” (ab)uses cellular pathways to optimize acquisition by the vector, and this long before arrival of the latter on an infected plant.
A lot of questions remain open, though. Are P2 and the other components of the transmission body actively transported on microtubules, or is their transient colocalization with microtubules part of an alternative transport mode? We started to more closely examine interaction between P2 and microtubules and privileged the hypothesis that the protein might be transported by a motor activity on microtubules. As preliminary data indicated that P2 does not possess an innate translocating activity, we looked for a cellular motor protein and tested as a candidate the kinesin TBK5.Citation12 This transport protein is, when overexpressed, able to bundle microtubules into a single focus, just as transmission bodies are singular structures in the cell. When healthy protoplasts were cotransfected with TBK5 and CaMV, TBK5 localized transiently with P2 on microtubules and in transmission bodies ( and D). This might be taken as the first evidence that a kinesin might be involved in formation of transmission bodies, but more experimentation is needed to confirm this hypothesis.
A by far more important question is: Have also other viruses, whether from the plant or the animal kingdoms, that are noncirculantly (or mechanically, as animal virologists call this mode of transmission) transmitted, developed similar strategies that fine-tune interactions between the host and the virus to prepare and perfect transmission?
Figures and Tables
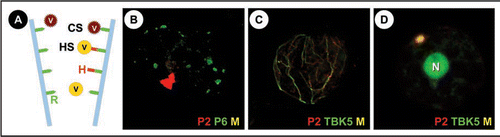
Figure 1 (A) The different strategies of non-circulant transmission: Viruses (V) using the capsid strategy (CS) attach directly to a receptor (R) in the tip of the a proboscis forming aphid stylets (blue), whereas in the helper strategy (HS) this interaction is mediated by the viral helper protein (H) that binds the virus particle to the receptor. Note that the helper protein can bind independently of the virus to the stylets. Whether the same receptor is used by different viruses as presented in the schema, is not known. (B) A turnip protoplast transfected with CaMV was double-labelled late in infection for CaMV helper protein P2 (red) and the marker protein for the virus factories P6 (green). It is visible that P2 localizes in a single, large transmission body, whereas the numerous virus factories are devoid of P2 (Colocalization would be revealed by yellowish color (M) in this superposition). (C and D) Turnip protoplasts were cotransfected with CaMV and TBK5-GFP and immunolabelled for P2 (red) and TBK5 was detected by GFP fluorescence. (C) shows a cell early in infection, where P2 and TBK5-GFP colocalize on a network that we identified as the microtubule cytoskeleton (unpublished data). (D) shows a cell later in infection where P2 and TBK5-GFP colocalize, as indicated by the yellowish color, in a transmission body. Note that TBK5-GFP also strongly labels the nucleus (N).
Addendum to:
References
- Martin B, Collar JL, Tjallingii WF, Fereres A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol 1997; 78:2701 - 2705
- Ziegler-Graff V, Brault V. Role of vector-transmission proteins. Meth Mol Biol 2008; 451:81 - 96
- Palacios I, Drucker M, Blanc S, Leite S, Moreno A, Fereres A. Cauliflower mosaic virus is preferentially acquired from the phloem by its aphid vectors. J Gen Virol 2002; 83:3163 - 3171
- Uzest M, Gargani D, Drucker M, Hébrard E, Garzo E, Candresse T, et al. A protein key to plant virus transmission at the tip of the insect vector stylet. Proc Natl Acad Sci USA 2007; 104:17959 - 17964
- Pirone T, Blanc S. Helper-dependent vector transmission of plant viruses. Annu Rev Phytopathol 1996; 34:227 - 247
- Armour SL, Melcher U, Pirone TP, Lyttle DJ, Essenberg RC. Helper component for aphid transmission encoded by region II of cauliflower mosaic virus DNA. Virology 1983; 129:25 - 30
- Drucker M, Froissart R, Hébrard E, Uzest M, Ravallec M, Espérandieu P, et al. Intracellular distribution of viral gene products regulates a complex mechanism of cauliflower mosaic virus acquisition by its aphid vector. Proc Natl Acad Sci USA 2002; 99:2422 - 2427
- Espinoza AM, Medina V, Hull R, Markham PG. Cauliflower mosaic virus gene II product forms distinct inclusion bodies in infected plant cells. Virology 1991; 185:337 - 344
- Nakayashiki H, Tsuge S, Kobayashi K, Okuno T, Furusawa I. Reasons for the low accumulation level of aphid transmission factor protein in infected leaves with an aphid-non-transmissible cauliflower mosaic virus isolate, CM1841. J Gen Virol 1993; 74:2469 - 2472
- Khelifa M, Journou S, Krishnan K, Gargani D, Espérandieu P, Blanc S, et al. Electron-lucent inclusion bodies are structures specialized for aphid transmission of cauliflower mosaic virus. J Gen Virol 2007; 88:2872 - 2880
- Haas M, Bureau M, Geldreich A, Yot P, Keller M. Cauliflower mosaic virus: still in the news. Mol Plant Pathol 2002; 3:419 - 429
- Goto Y, Asada T. Excessive expression of the plant kinesin TBK5 converts cortical and perinuclear microtubules into a radial array emanating from a single focus. Plant Cell Physiol 2007; 48:753 - 761