Abstract
We recently reported that autophagy plays a role in chloroplasts degradation in individually-darkened senescing leaves. Chloroplasts contain approximately 80% of total leaf nitrogen, mainly as photosynthetic proteins, predominantly ribulose 1, 5-bisphosphate carboxylase/oxygenase (Rubisco). During leaf senescence, chloroplast proteins are degraded as a major source of nitrogen for new growth. Concomitantly, while decreasing in size, chloroplasts undergo transformation to non-photosynthetic gerontoplasts. Likewise, over time the population of chloroplasts (gerontoplasts) in mesophyll cells also decreases. While bulk degradation of the cytosol and organelles is mediated by autophagy, the role of chloroplast degradation is still unclear. In our latest study, we darkened individual leaves to observe chloroplast autophagy during accelerated senescence. At the end of the treatment period chloroplasts were much smaller in wild-type than in the autophagy defective mutant, atg4a4b-1, with the number of chloroplasts decreasing only in wild-type. Visualizing the chloroplast fractions accumulated in the vacuole, we concluded that chloroplasts were degraded by two different pathways, one was partial degradation by small vesicles containing only stromal-component (Rubisco containing bodies; RCBs) and the other was whole chloroplast degradation. Together, these pathways may explain the morphological attenuation of chloroplasts during leaf senescence and describe the fate of chloroplasts.
The most abundant chloroplast protein is Rubisco, comprising approximately 50% of the soluble protein.Citation1 The amount of Rubisco decreases rapidly in the early phase of leaf senescence, and more slowly in the later phase. During senescence, chloroplasts gradually shrink and their numbers gradually decrease in mesophyll cells.Citation2,Citation3 During leaf senescence, leaves lose approximately 75% of their Rubisco, while chloroplast numbers decrease by only about 15%.Citation4 Previous studies showed chloroplasts localized within the central vacuole by electron microscopy, indicating chloroplast degradation in the highly hydrolytic vacuole.Citation5 However, there was no direct evidence showing translocation of chloroplasts from the cytosol to the vacuole, and the mechanism of transportation was also unclear.
Recent reverse genetic approaches are helping to elucidate the autophagy system in plants, which has a similar molecular mechanism as in yeast.Citation6–Citation11 In Arabidopsis (Arabidopsis thaliana), atg mutants have phenotypically accelerated leaf senescence, insufficient root elongation in nutrient starvation condition and reduced seeds yields, therefore, autophagy is considered to be important for nutrient recycling especially nutrient starvation and senescence in plants.Citation12
In Arabidopsis, individually darkened rosette leaves (IDLs) exhibit enhanced senescence.Citation13 Appling IDLs treatment as an experimental model of leaf senescence, we recently demonstrated that chloroplasts are degraded in two different pathways by autophagy, one for RCBs,Citation14,Citation15 and one for whole chloroplast.Citation16 Darkened leaves became pale in 3 to 5 days treatment, while illuminated parts normally grow in both wild-type and autophagy defective mutant, atg4a4b-1. Furthermore, genes specifically expressed during senescence, SAG12 and SEN1, were rapidly upregulated, meanwhile, photosynthetic genes, such as RBCS2B and CAB2B, were gradually downregulated. All analyzed ATG genes were also upregulated under IDL treatment, which suggests that autophagy is important in IDL senescence. It has been reported that approximately three quarter genes of upregulated in IDL were also upregulated in naturally senescing leaves, including the ATG genes.Citation17 This suggests that the autophagy pathways used in IDLs are also used in naturally senescing leaves.
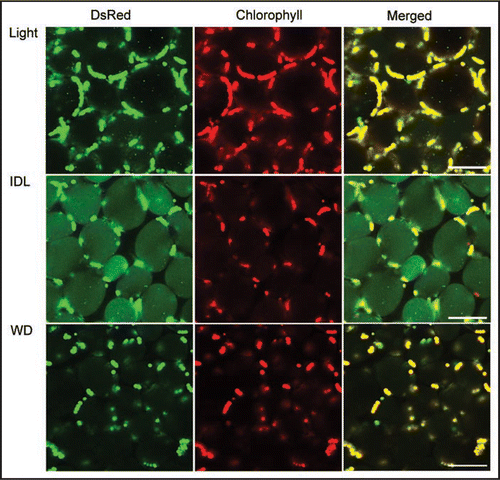
Over the 5 day treatment period, chloroplasts of wild-type IDL shrink to approximately one third their original size. In atg4a4b-1, by contrast, chloroplasts shrinkage occurred immediately after the start of IDL treatment after which no further shrinkage was noted. While the shrunk chloroplasts in fixed cells of wild-type were still smooth and round, while wrinkly chloroplasts were observed in atg4a4b-1. At same time, in the living mesophyll cells of wild-type IDL, RCBs accumulated in the vacuole (). The shrinkage of chloroplasts may be due to the consumption of the chloroplast envelope by RCB formation. Immunological quantification of inner and outer envelope proteins might confirm this hypothesis. The chloroplast number was also gradually decreased in IDL of wild-type plants, but no decline in chloroplast number was noted in atg4a4b-1. Chloroplasts exhibiting chlorophyll auto-fluorescence were found in the vacuole of wild-type IDLs, but not in atg4a4b-1 IDLs. These results show that whole chloroplast degradation is also performed by autophagy. However, the transport pathway of whole chloroplasts into the vacuole remains unclear. The chloroplast, even in its shrunken state, is a large organelle, and the autophagosome, the carrier bodies of autophagy, which usually target small spherical organelles like mitochondria and peroxisomes, may be incapable of isolating large organelles. In the yeast autophagy system, specific cellular organelles and fractions are also transported via vacuolar membrane invagination using the microautophagy system.Citation18 RCB uptake into the vacuole is termed macroautophagy, while larger organelles, such as chloroplasts, are engulfed in a process known as microautophagy. Whether there exists a molecular difference between these processes, or whether this is an arbitrary division based solely on the size of the consumed body is unclear.
Whole darkened plants exhibit retarded leaf aging, in contrast to the accelerated senescence in IDLs.Citation13 Whole darkened plants suppress leaf senescence with the leaves retaining green color. After 5 days, in the mesophyll cells of whole darkened plants, any translocation of chloroplast components, stroma-targeted DsRed, RCBs, and whole chloroplasts, into the vacuole could hardly be detected (). This suggests that autophagy is not induced by darkness alone, and is associated closely with senescence. ATG genes were downregulated in the whole darkened wild-type plants less than control plants during the treatment. Previous studies have shown that following about 5 day period of whole plant darkening, atg mutants lose their ability to protect themselves against photo-damage.Citation7 Upon return to the light, these plant quickly undergo terminal photo-bleaching.
Concentrations of chlorophyll, soluble protein, leaf nitrogen and Rubisco rapidly declined under IDL condition of both wild-type and atg4a4b-1. Considering the accumulated fluorescence of stroma-targeted Ds-Red in the vacuole and autophagy dependent size shrinkage of chloroplasts in IDL, in wild-type plants RCB autophagy appear to be responsible for a sizable proportion of chloroplast protein degradation. In atg4a4b-1 which cannot form RCBs, alternative degradation pathways must be upregulated, with chloroplast proteases the most likely candidates. Intriguingly, the decrease in Rubisco concentration proceeds at the almost identical rates in both wild-type and atg4a4b-1 plants, despite the different degradation pathways. It seems likely that the rate of Rubisco degradation may be regulated at an early step in the degradation pathway, by some, as yet unknown, factors.
Chloroplasts appear to have the ability to control their volume during cell division, dividing and increasing their density up to the certain level,Citation19 and transferring their cellular components between them via stromules.Citation20 How chloroplasts are able to regulate their volume remains unclear, but it seems likely that chloroplasts grow and divide, like any other bacteria, as long as sufficient resources remain in the environment, in this case the cell. Total chloroplast volume, therefore, may be limited by the availability of carbon, nitrogen, or other nutrients in the cell during leaf emergence. Chloroplasts may be also able to reduce and control their volumes during leaf senescence via multiple degradation pathways. Our next goal is to estimate the contribution of both RCBs and whole chloroplasts autophagy in chloroplast protein degradation during natural leaf senescence. Further investigations are required for understanding the specific molecular mechanisms of RCB production and whole chloroplast degradation.
Figures and Tables
Figure 1 Visualization of stroma-targeted DsRed and chlorophyll autofluorescence in living mesophyll cells of wild-type plants by laser-scanning confocal microscopy. A excised control leaf (A, Light) and an individually darkened leaf (B, IDL) from plants grown under 14 h-photoperiod condition and a leaf from whole-plant darkened condition (WD, C) for 5days were incubated with 1 µM concanamycin A in 10 mM MES-NaOH (pH 5.5) at 23C° for 20 h in darkness. Stroma-targeted DsRed appears green and chlorophyll fluorescence appears red. In merged images, overlap of DsRed and chlorophyll fluorescence appears yellow. Small vesicles with stromal-targeted DsRed, i.e. RCBs, can be found in the vacuole (A, B). In IDL (B), massive accumulation of stroma-targeted DsRed is entirely seen in the vacuolar lumen and chloroplasts losing DsRed fluorescence are found in some cells. Bars = 50 µm.
Acknowledgements
We thank Dr. Louis Irving for critical reading of the manuscript. This research was supported by KAKENHI (nos. 19039004, 20200061 and 20780044) to H.I.
Addendum to:
References
- Wittenbach VA. Breakdown of ribulose bisphosphate carboxylase and change in proteolytic activity during dark-induced senescence of wheat seedlings. Plant Physiol 1978; 62:604 - 608
- Ono K, Hashimoto H, Katoh S. Changes in the number and size of chloroplasts during senescence of primary leaves of wheat grown under different conditions. Plant Cell Physiol 1995; 36:9 - 17
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T. Three-dimentional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles—investigations of tissues and cells by fluorescence microscopy. Planta 205:142 - 155
- Mae T, Kai N, Makino A, Ohira K. Relation between ribulose bisphosphate carboxylase content and chloroplast number in naturally senescing primary leaves of wheat. Plant Cell Physiol 1984; 25:333 - 336
- Wittenbach VA, Lin W, Hebert RR. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol 1982; 69:98 - 102
- Yoshimoto K, Hanaoka H, Sato S, Kato T, Tabata S, Noda T, et al. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 2004; 16:2967 - 2983
- Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol 2005; 138:2097 - 2110
- Xiong Y, Contento AL, Bassham DC. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J 2005; 42:535 - 546
- Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumer SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005; 121:567 - 577
- Fujiki Y, Yoshimoto K, Ohsumi Y. An Arabidopsis homolog of yeast ATG6/VPS30 is essential for pollen germination. Plant Physiol 2007; 143:1132 - 1139
- Phillips AR, Suttangkakul A, Vierstra RD. The ATG12 conjugating enzyme ATG10 is essential for autophagic vesicle formation in Arabidopsis thaliana. Genetics 2008; 178:1339 - 1353
- Thompson AR, Vierstra RD. Autophagic recycling: lessons from yeast help define the process in plants. Curr Opin Plant Biol 2005; 8:165 - 173
- Weaver LM, Amasino RM. Senescence is induced in individually darkened Arabidopsis leaves but inhibited in whole darkened plants. Plant Physiol 2001; 127:876 - 886
- Chiba A, Ishida H, Nishizawa NK, Makino A, Mae T. Exclusion of ribulose-1,5-bisphosphate carboxylase/oxygenase from chloroplasts by specific bodies in naturally senescing leaves of wheat. Plant Cell Physiol 2003; 44:914 - 921
- Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, et al. Mobilization of Rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagy process. Plant Physiol 2008; 148:142 - 155
- Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, et al. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol 2009; 149:885 - 893
- van der Graaff E, Schwache R, Schneider A, Desimone M, Flügge UI, Kunze R. Transcription analysis of Arabidopsis membrane transports and hormone pathways during developmental and induced leaf senescence. Plant Physiol 2006; 141:776 - 792
- Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med 2003; 9:65 - 76
- Pyke KA, Leech RM. A genetic analysis of chloroplast devision and expansion in Arabidopsis thaliana. Plant Physiol 1994; 104:201 - 207
- Köhler RH, Cao J, Zipfel WR, Webb WW, Hanson MR. Exchange of protein molecules through connections between higher plant plastids. Science 1997; 276:2039 - 2042