Abstract
The unripe fruits of certain species are red. Some of these species disperse their seeds by wind (Nerium oleander, Anabasis articulata), others by adhering to animals with their spines (Emex spinosa) or prickles (Hedysarum spinosissimum). Certainly neither type uses red coloration as advertisement to attract the seed dispersing agents. Fleshy-fruited species (Rhamnus alaternus, Rubus sanguineus and Pistacia sp.), which disperse their seeds via frugivores, change fruit color from green to red while still unripe and then to black or dark blue upon ripening. The red color does not seem to function primarily in dispersal (unless red fruits form advertisement flags when there are already black ripe fruits on the plant) because the red unripe fruits of these species are poisonous, spiny, or unpalatable. The unripe red fruits of Nerium oleander are very poisonous, those of Rhamnus alaternus and Anabasis articulata are moderately poisonous, those of Rubus sanguineus are very sour, those of Pistacia sp. contain unpalatable resin and those of Emex spinosa and Hedysarum spinosissimum are prickly. We propose that these unripe red fruits are aposematic, protecting them from herbivory before seed maturation.
Introduction
Advertisement is an important aspect of plant-animal relationships because it attracts pollinators to flowersCitation1–Citation4 and seed-dispersing frugivores to ripe fruit.Citation5–Citation10 Fruit color is the typical visual signal that plants use to communicate with frugivores,Citation5,Citation6,Citation8,Citation11–Citation14 although other plant parts (branches of the raceme, panicle or leaves) may also contribute to this signaling system.Citation5,Citation7
Multicolored fruit displays, where fruits first change their color from green to a conspicuous color when they have reached full size but are still unripe and later to a second conspicuous color upon ripening, have been studied in plants of several ecosystems.Citation7,Citation8,Citation11,Citation15–Citation21 Some studies indicated that bicolored fruit displays enhance seed dispersalCitation7,Citation11,Citation15–Citation19,Citation21–Citation23 but other studies did not.Citation11,Citation15,Citation16,Citation19,Citation23 Thus, it seems that promotion of dispersal can only partly explain multicolored fruit displays.
Color change is a very well known and widely distributed phenomenon in flowers.Citation4,Citation24,Citation25 In many plants, old or pollinated flowers are retained to continue their contribution to the advertisement of the whole plant or inflorescence even after they stop producing nectar and change their color. As a result, pollinators are attracted to the inflorescences but when approaching the flowers they prefer the young rewarding pre-color-change flowers to the old, unrewarding ones.Citation4,Citation24 In such a way the whole plant retains its long-range advertisement without reducing the pollination probability of its young and virgin flowers. Multicolored fruit displays seem to be a convergent phenomenon, where full size unripe colored fruits are a part of the general advertisement, but approaching frugivores prefer the more palatable and rewarding ripe fruits that have changed their color.Citation7,Citation21,Citation22 Color changes are a broad phenomenon in youngCitation26,Citation27 and senescing leaves,Citation28–Citation31 and these color changes were proposed to serve physiological functions (see refs. Citation32 and Citation33), anti herbivory functions (see refs. Citation29, Citation34 and Citation35) or both (see refs. Citation36–Citation38). Color changes also characterize old aposematic spines, thorns and prickles of many taxa that lose their conspicuousness when the organ they protect matures and needs less defense.Citation39
Aposematic coloration, a well-known phenomenon in animals (e.g., see refs. Citation40–Citation42), has recently been shown to be common also in thorny plants and in plants that mimic themCitation39,Citation42–Citation57 as well as in certain poisonous plants, some of which proposed to be aposematic earlier.Citation13,Citation14,Citation36–Citation38,Citation46,Citation47,Citation51,Citation58–Citation67 In animals, aposematic coloration is usually red, orange, yellow, black and white or combinations of these, which protect unpalatable, dangerous or poisonous prey species.Citation40–Citation42 The similarity between aposematic colors in poisonous insects and ripe fruits has already been demonstrated,Citation68,Citation69 but their aposematic role in fruits was not considered by HerreraCitation68 and was not found to operate in artificial fruit-like objects introduced to domestic chicks.Citation69 Several types of defensive aposematic coloration have been proposed to occur in fruits that repel large herbivores: (1) brightly colored poisonous fruits;Citation13,Citation14,Citation60,Citation65 (2) pods of several wild annual legumes (Lathyrus ochrus, Pisum humile, P. elatius and Vicia peregrina) have conspicuous reddish spots arranged along them that appear to mimic aposematic lepidopteran caterpillars and may repel various herbivores;Citation37,Citation46,Citation47,Citation70 (3) colorful (yellow, red, purple or various combinations of these) aposematic thorn-like unripe soft fruits in several wild Erodium species and in Sinapis alba growing in Israel.Citation44
Here we report that conspicuous red but unripe fruit of several plant species are poisonous or unpalatable. We propose that in such cases fruit color may contribute to the general attraction of frugivores to the plant but at the same time serve as an aposematic signal that deters herbivores and frugivores from the dangerous or unpalatable unripe fruit. Thus, colorful aposematic unripe fruits may increase plant fitness by deterring herbivores and frugivores from consuming fruits, including their immature seeds.
Results
The species of the Israeli flora with unripe poisonous or unpalatable red fruits are described below.
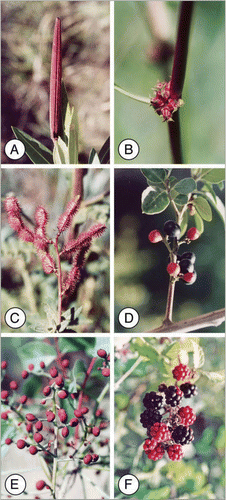
Nerium oleander L., is a tall evergreen multi-stemmed shrub or small tree (Apocynaceae), common along seasonal streams, rivers and in other wet habitats. Its fruit (5–15 cm long and 6–10 mm in diameter) consists of two follicles. The young unripe small fruit is green but it turns red when fully grown but still unripe (). Upon maturation, the fruit dries, turns brown and its follicles twist to liberate the many small, plumed, wind-dispersed seeds.Citation71 The whole plant, including its fruit, is extremely toxic, containing about 2% cardiac glycosides.Citation72 More than ten glycosides with known chemical structure were reported from N. oleander. The cardiac effects of the glycosides are due to direct cardiotoxicity and an indirect effect via the vagal nerve. The direct effect is due to the inhibition of the Na-K ATP-ase pump enzyme system. This specific action increases intracellular sodium ions and serum potassium concentration.Citation73 The lethal dose of leaves reported for several animal species is about 0.5 mg per 1 kg body mass.Citation74
Emex spinosa (L.) Campd. is an annual plant (Polygonaceae), common in semi-arid open areas and grasslands. Its fully grown unripe fruits are red and spiny (). When they dry, their spines turn light brown and stick to the fur of passing animals, thereby transferring the seeds over distances.
Hedysarum spinosissimum L. [H. pallens (Moris) Halacsy] is an annual plant (Fabaceae), typical of sandy soils of the steppe. Red prickles cover its unripe pods (), which turn light brown when ripe and stick to the fur of passing animals, transferring the ripe seeds.
Rhamnus alaternus L. is a tall evergreen shrub or small tree (Rhamnaceae), common in the Mediterranean maquis. Its young small fruits are green and turn red when fully grown but still unripe (). The red fruits contain 7.8 ± 2.8 ppm of the anthraquinone emodin and are poisonous to some extent. This concentration decreases to 2.5 ± 0.9 ppm in ripe black fruits.Citation75 Similar fruit color change also occurs in R. lycioides L., in R. punctata Boiss., and probably also in other Rhamnus species.
Species of the woody genus Pistacia (e.g., the trees P. atlantica Desf. and P. palaestina Boiss., and the shrub P. lentiscus L.), grow in various habitats in the Mediterranean, steppe or in the case of P. atlantica even in desert districts. In Pistacia spp. the young green fruits turn red (or yellow in some P. lentiscus individuals) when fully grown but still unripe with immature seeds, and are unpalatable ().Citation76 Later, upon fruit ripening and seed maturation, the fruits turn dark blue or black. Seed ripening as well as structural and chemical changes in the fruit pulp accompany this color change. The fruit softens, the concentrations of its secondary repellent compounds decrease while that of lipids and proteins increase, and it turns palatable to frugivores.Citation76–Citation78 The red immature fruits are less rewarding and less palatable than the mature dark ones; consequently, frugivorous birds prefer to consume dark fruits and not red ones.Citation23 Pistacia has also typical red seedless fruits that are not aborted but retained on the tree. These empty fruits contribute to the visual attraction of fruit-bearing trees and shrubs, but are consumed by frugivores only when the dark seed containing fruits are not available.Citation23,Citation78,Citation79
Rubus sanguineus Friv., (R. sánctus Schreb.) (blackberry) (Rosaceae) is a thorny climber, often with a shrubby form, common along streams and in other wet habitats. Compound drupes carried in groups characterize blackberries (Rubus spp.). The color of young small fruits is green. After an initial growth period, they reach their final size and turn bright red (). However, at this stage, the fruit is still unripe and very sour. Many times, the conspicuous groups of unripe red compound drupes dominate the plants. Only later in season, when the fruits turn black and sweet () frugivorous birds consume them and disperse the seeds.Citation80,Citation81 We measured the sugar content of fruits at the three color stages. The green fruits contained 7.5%, the red 9% and the black 20% sugars. In parallel, the acidity decreased from pH 2.6–2.9 in green and red unripe fruit to pH 3.6 in black ripe fruits.
Anabasis articulata (Forssk.) Moq. is a poisonous shrub (Chenopodiaceae), common in the Negev desert. Its unripe winged fruits are purple, red or pink. After ripening, they are wind dispersed. The plant is poisonous, rich in alkaloids and is not eaten by grazers.Citation51,Citation82,Citation83
Discussion
In spite of the numerous studies dealing with the ecology and evolution of fruit color, the hypothesis that aposematic unripe red fruits protect their seeds has attracted only a little theoretical attention,Citation37,Citation46,Citation47,Citation60–Citation62,Citation70 a single experimental behavioral studyCitation65 and a theoretical discussion based on detailed measurements of fruit chemistry and color and of bird visual system.Citation14 The poisonous or otherwise unpalatable species presented here, which have unripe red fruits and the two other types of defensive aposematic coloration (mimicry of caterpillars and spines), which have recently been proposed to occur in fruits and repel large herbivoresCitation44,Citation47,Citation70 seem to represent a widespread but still almost unexplored phenomenon. The aposematic unripe fruit hypothesis is further supported by the fact that birds detect red fruits from a longer distance than black fruits.Citation84
Nerium oleander disperses its seeds by wind, and the red advertisement of the unripe and very poisonous fruits certainly does not promote seed dispersal. The combination of red fruits with their very poisonous latex (see refs. Citation71, Citation72 and Citation85) is sufficient to characterize their color as aposematic. The fact that the seeds are dispersed by wind further demonstrates the probable deterring function of the red color rather than advertisement for potential seed dispersers. Similarly, the wind-dispersed poisonous purple, red or pink fruits of Anabasis articulata need no advertisement to promote seed dispersal and thus may also be characterized as aposematic. The spiny unripe red fruits of Emex spinosa and Hedysarum spinosissimum are also unpalatable for large mammalian herbivores and need no advertisement to attract frugivores because they are epizoochorous, with dispersal units that adhere to animals' fur, hooves or feathers. Therefore, the red color of their fruits can also be regarded as aposematic.
The fleshy-fruited species (Rhamnus alaternus and other Rhamnus species and Rubus sanguineus) disperse their seeds via frugivores only after they change color from red to black. The red fruits of Rhamnus alaternus are poisonous and those of Rubus sanguineus are very sour and low in sugar content at their red unripe stage, but sweet and not sour when black and fully ripe. It has been demonstrated for the Old World's Rhamnus alaternus and R. lycioides (R. palaestinus) and for the New World's R. cathartica that bird species do not consume unripe red fruits.Citation75,Citation86,Citation87 Emodin is the predominant secondary metabolite in Rhamnus fruits and recently it has been demonstrated that birds and small mammals are unable to detoxify high concentrations of emodin efficiently.Citation88 There are good indications for considerable predispersal seed predation in R. alaternus,Citation89 which may trigger the evolution of defense.
In the bird-dispersed Rhamnus alaternus, Rubus sanguineus and Pistacia sp. the fruits have three color stages. The first, green stage is common to all unripe small fruits. At this stage the fruit is cryptic and photosynthetically active, contributing to its own production costs and defended by both crypsis and unpalatability. Producing and bearing non-green, fully grown yet unripe fruits for substantial periods, adds to the costs of fruit production. Such a trait is supposed to be compensated for by another contribution to the fitness of the plant. Increasing the long-range advertisement of a fruit-bearing plant and its consequent attractiveness to frugivores, which also increases the probability of ripe fruit removal, can provide such compensation. Indeed, it has been demonstrated that red unripe or empty fruits of Pistacia contribute to the removal rate of the black ripe fruits by increasing the attraction of frugivores.Citation78
Our proposal that unripe red fruits are aposematic, contributes to the understanding of fruit color change and does not contradict any other explanation. Color change in fruits can be regarded as analogous to color change of flowersCitation4,Citation24 and to color changes in aposematic thorn, spine and prickle.Citation39 Pre- and post-color change organs contribute to the overall attraction of pollinators and frugivores, or repulsion of herbivores. However, when approaching the plant, pollinators visit the pre-color change rewarding flowers, while frugivores turn to the post-color-change palatable, rewarding, non-poisonous fruits. This strengthens the concept that color itself has only a limited meaning unless coupled with reward or punishment. Thus, red color can be attractive when related to rewarding fruits or aposematic when related to poisonous, sour, bitter or spiny fruits. Similarly, floral color and odor function as pollinator attractorsCitation2 when coupled with reward, but there is also good evidence for their defensive function.Citation90–Citation92 HintonCitation59 proposed that bright colors of poisonous flowers are not only attractive, but also aposematic. The duality of signaling systems, serving in attracting certain animals and repelling others at the same time did not get much research attention. Pollen odors in certain wind-pollinated plants that do not attract pollinators are rich with defensive molecules such as α-methyl alcohols and ketones.Citation93 The de-aromatized isoprenylated phloroglucinols may visually attract pollinators of Hypericum calycinum by their UV pigmentation properties, but at the same time the plant may use this pigmentation as a toxic substance against caterpillars, defending the flowers from herbivores.Citation94 Herrera et al.Citation95 proposed that plants that possess a particular combination of traits that simultaneously enhance pollination and defend from herbivores enjoy a disproportionate higher fitness advantage over plants possessing individual traits of such combinations. The double action of attracting pollinators while deterring other animals was found in flowers of other taxa (e.g., see refs. Citation92 and Citation96–Citation99), a principle that may also be true for fruit color.
Proving that certain fruits are poisonous or unpalatable is not an easy task because some frugivores consume fruits that are unpalatable or poisonous to others.Citation100,Citation101 We propose that the red color of poisonous or unpalatable unripe fruits might serve as an aposematic deterring signal for frugivores. The data presented here support previous proposals of aposematism in fruits.Citation13,Citation14,Citation37,Citation44,Citation46,Citation47,Citation62,Citation63,Citation65,Citation70 The possible benefits for the signaler plant are: (1) increase in the advertisement of the fruit-bearing plant and attraction from longer distances of more frugivores per fruit unit;Citation7,Citation17,Citation18,Citation21,Citation22 (2) reduction in the consumption of immature seeds; (3) reduction in the abortion of damaged fruits, before seed maturation, because wounding of fruits can stimulate ethylene production and consequent abscission;Citation102 (4) reduction in the possible damage to fruits, which might later decrease seed dispersal because frugivores avoid eating damaged fruits (especially in large fruits).Citation103–Citation109 The frugivores benefit from the signal because: (1) they avoid poisonous fruits that contain harmful substances or spiny ones, and (2) they refrain from damaging future fruit resources in their home range.
We have many good reasons to think that physiological benefits may also be involved in red fruit coloration, such as the protection by anthocyanins from photoinhibition and photooxidation.Citation33,Citation110–Citation112 Gould et al.Citation110 Lev-Yadun et al.Citation113,Citation114 Lev-Yadun,Citation46,Citation47 Lev-Yadun and GouldCitation36,Citation37 and Archetti et al.Citation38 have already argued that the non-photosynthetic plant pigments have the potential to serve more than one function concurrently. Thus, various hypotheses concerning coloration of fruits need not contrast or exclude any other functional explanation. Moreover, fruit color change might have more than one type of benefit and be selected for by several agents.
We conclude that there are certain unripe poisonous or spiny red colored fruits that are probably aposematic, as demonstrated from the flora of Israel. We propose that this is probably a common but largely overlooked worldwide syndrome. As with other color-dependent defensive strategies, we expect that aposematic red fruits were subjected to mimicry by young unripe red fruits that contain no poison.
Materials and Methods
Following the recent discussions that many colorful thorny or poisonous plants might be aposematicCitation36–Citation39,Citation42–Citation53,Citation55–Citation57,Citation66,Citation67 and that several colorful fruits are aposematic because they are poisonous,Citation13,Citation14,Citation47,Citation60–Citation62,Citation65 mimic poisonous caterpillars,Citation37,Citation46,Citation70 or mimic aposematic colorful spines,Citation37,Citation44,Citation46,Citation47 a new hypothesis should be considered, namely, that unripe red fruits might also be aposematic. To explore this issue, we have screened the plants in the flora of Israel that have unripe red fruits and discuss their potential of being aposematic. For one species, Rubus sanguineus Friv., (R. sánctus Schreb.) (blackberry) of the Rosaceae, we also measured the sugar content of the fruits at three color stages (green, red and black) using a refractrometer (Atago ATC-1E, 0%–32%).
Figures and Tables
Figure 1 (A) Red unripe poisonous fruit of Nerium oleander. (B) The spiny unripe red fruits of Emex spinosa. (C) Red prickles cover the unripe pods of Hedysarum spinosissimum. (D) Poisonous unripe red fruits and a ripe black fruit of Rhamnus alaternus. (E) Red unripe fruits of Pistacia palaestina. (F) Red sour unripe fruits and sweet black ripe fruits of Rubus sanguineus.
References
- Darwin C. The different forms of flowers on plants of the same species 1877; London, GB John Murray
- Faegri K, Pijl van der L. The principles of pollination ecology 1979; 3rd ed. Oxford, UK Pergamon Press
- Willson MF. Plant reproductive ecology 1983; New York, NY John Wiley & Sons
- Weiss MR. Floral colour change: a widespread functional convergence. Am J Bot 1995; 82:167 - 195
- Ridley HN. The dispersal of plants throughout the world 1930; Ashford, GB L. Reeve & Co. Ltd
- Pijl van der L. Principles of dispersal in higher plants 1982; 3rd ed. Berlin, Germany Springer-Verlag
- Stiles EW. Fruit flags: Two hypotheses. Am Nat 1982; 120:500 - 509
- Willson MF, Whelan CJ. The evolution of fruit color in fleshy-fruited plants. Am Nat 1990; 136:790 - 809
- Schmidt V, Schaefer HM, Winkler H. Conspicuousness, not colour as foraging cue in plant-animal signalling. Oikos 2004; 106:551 - 557
- Schaefer HM, Schaefer V, Vorobyev M. Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals?. Am Nat 2007; 169:S159 - S169
- Janson CH. Bird consumption of bicolored fruit displays. Am Nat 1987; 130:788 - 792
- Snow B, Snow D. Birds and berries. A study of an ecological interaction 1988; Calton, UK T. & A.D. Poyser
- Schaefer HM, Schmidt V. Detectability and content as opposing signal characteristics in fruits. Proc Roy Soc Lond B 2004; 271:S370 - S373
- Schaefer HM, Schaefer V. Dennis AJ, Schupp EW, Green R, Wescott DW. The evolution of visual fruit signals: Concepts and constraints. Seed dispersal: theory and its application in a changing world 2007; Wallingford, UK CAB International 59 - 77
- Morden-Moore AL, Willson MF. On the ecological significance of fruit color in Prunus serotina and Rubus occidentalis: field experiments. Can J Bot 1982; 60:1554 - 1560
- Willson MF, Thompson JN. Phenology and ecology of color in bird-dispersed fruits, or why some fruits are red when they are “green”. Can J Bot 1982; 60:701 - 713
- Willson MF, Melampy MN. The effect of bicolored fruit displays on fruit removal by avian frugivores. Oikos 1983; 41:27 - 31
- Wheelwright NT, Janson CH. Colors of fruit displays of bird-dispersed plants in two tropical forests. Am Nat 1985; 126:777 - 799
- Fuentes M. The effect of unripe fruits on ripe fruit removal by birds in Pistacia terebinthus: flag or handicap?. Oecologia 1995; 101:55 - 58
- Traveset A, Willson MF. Ecology of the fruit-colour polymorphism in Rubus spectabilis. Evol Ecol 1998; 12:331 - 345
- Cramer JM, Cloud ML, Muchhala NC, Ware AE, Smith BH, Williamson GB. A test of the bicolored fruit display hypothesis: Berry removal with artificial fruit flags. J Torrey Bot Soc 2003; 130:30 - 33
- Greig-Smith PW. Bicolored fruit displays and frugivorous birds: the importance of fruit quality to dispersers and bird predators. Am Nat 1986; 127:246 - 251
- Izhaki I. The relationships between fruit ripeness, wasp seed predation, and avian fruit removal in Pistacia palaestina. Isr J Plant Sci 1998; 46:273 - 278
- Weiss MR, Lamont BB. Floral colour change and insect pollination: a dynamic relationship. Isr J Plant Sci 1997; 45:185 - 199
- Ne`eman G, Nesher R. Pollination ecology and the significance of colour change in Lupinus pilosus L. Isr J Plant Sci 1995; 43:135 - 145
- Richards PW. The tropical rain forest an ecological study 1996; 2nd ed. Cambridge, UK Cambridge University Press
- Dominy ND, Lucas PW, Ramsden W, Riba-Hernandez P, Stoner KE, Turner IM. Why are young leaves red?. Oikos 2002; 98:163 - 176
- Matile P. Biochemistry of Indian summer: physiology of autumnal leaf coloration. Exp Gerontol 2000; 35:145 - 158
- Archetti M. The origin of autumn colours by coevolution. J Theor Biol 2000; 205:625 - 630
- Archetti M. Phylogenetic analysis reveals a scattered distribution of autumn colours. Ann Bot 2009; 103:703 - 713
- Hoch WA, Zeldin EL, McCown BH. Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol 2001; 21:1 - 8
- Lee DW. Anthocyanins in autumn leaf senescence. Adv Bot Res 2002; 37:147 - 165
- Ougham HJ, Morris P, Thomas H. The colors of autumn leaves as symptoms of cellular recycling and defenses against environmental stresses. Curr Top Dev Biol 2005; 66:135 - 160
- Hamilton WD, Brown SP. Autumn tree colours as a handicap signal. Proc R Soc Lond B 2001; 268:1489 - 1493
- Archetti M, Brown SP. The coevolution theory of autumn colours. Proc R Soc Lond B 2004; 271:1219 - 1223
- Lev-Yadun S, Gould KS. What do red and yellow autumn leaves signal?. Bot Rev 2007; 73:279 - 289
- Lev-Yadun S, Gould KS. Gould KS, Davies KM, Winefield C. Role of anthocyanins in plant defense. Life's colorful solutions: the biosynthesis, functions, and applications of anthocyanins 2008; Berlin, Germany Springer-Verlag 21 - 48
- Archetti M, Döring TF, Hagen SB, Hughes NM, Leather SR, Lee DW, et al. Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends Ecol Evol 2009; 24:166 - 173
- Lev-Yadun S, Ne`eman G. Color changes in old aposematic thorns, spines, and prickles. Isr J Plant Sci 2006; 54:327 - 333
- Cott HB. Adaptive coloration in animals 1940; London, GB Methuen & Co Ltd
- Edmunds M. Defence in animals. A survey of anti-predator defences 1974; Harlow, UK Longman Group Ltd
- Ruxton GD, Sherratt TN, Speed MP. Avoiding attack. The evolutionary ecology of crypsis, warning signals & mimicry 2004; Oxford, UK Oxford University Press
- Lev-Yadun S. Aposematic (warning) coloration associated with thorns in higher plants. J Theor Biol 2001; 210:385 - 388
- Lev-Yadun S. Weapon (thorn) automimicry and mimicry of aposematic colorful thorns in plants. J Theor Biol 2003; 244:183 - 188
- Lev-Yadun S. Why do some thorny plants resemble green zebras?. J Theor Biol 2003; 244:483 - 489
- Lev-Yadun S. Teixeira da Silva JA. Defensive coloration in plants: a review of current ideas about anti-herbivore coloration strategies. Floriculture, ornamental and plant biotechnology: advances and topical issues 2006; IV:London, UK Global Science Books 292 - 299
- Lev-Yadun S. Baluska F. Aposematic (warning) coloration in plants. Plant-environment interactions. From sensory plant biology to active plant behavior 2009; Berlin, Germany Springer-Verlag 167 - 202
- Lev-Yadun S. Müllerian and Batesian mimicry rings of white-variegated aposematic spiny and thorny plants: a hypothesis. Isr J Plant Sci 2009; In press.
- Lev-Yadun S. Müllerian mimicry in aposematic spiny plants. Plant Signal Behav 2009; 4:482 - 483
- Midgley JJ, Botha MA, Balfour D. Patterns of thorn length, density, type and colour in African Acacias. Afr J Range Forage Sci 2001; 18:59 - 61
- Lev-Yadun S, Ne`eman G. When may green plants be aposematic?. Biol J Linn Soc 2004; 81:413 - 416
- Midgley JJ. Why are spines of African Acacia species white?. Afr J Range Forage Sci 2004; 21:211 - 212
- Rubino DL, McCarthy BC. Presence of aposematic (warning) coloration in vascular plants of southeastern Ohio. J Torrey Bot Soc 2004; 131:252 - 256
- Speed MP, Ruxton GD. Warning displays in spiny animals: one (more) evolutionary route to aposematism. Evolution 2005; 59:2499 - 2508
- Halpern M, Raats D, Lev-Yadun S. Plant biological warfare: Thorns inject pathogenic bacteria into herbivores. Environ Microbiol 2007; 9:584 - 592
- Halpern M, Raats D, Lev-Yadun S. The potential anti-herbivory role of microorganisms on plant thorns. Plant Signal Behav 2007; 2:503 - 504
- Lev-Yadun S, Halpern M. Van Dijk T. External and internal spines in plants insert pathogenic microorganisms into herbivore's tissues for defense. Microbial ecology research trends 2008; New York, NY Nova Scientific Publishers Inc 155 - 168
- Cook AD, Atsatt PR, Simon CA. Doves and dove weed: multiple defenses against avian predation. BioScience 1971; 21:277 - 281
- Hinton HE. Gregory RL, Gombrich EH. Natural deception. Illusion in nature and art 1973; London, UK Duckworth 97 - 159
- Wiens D. Mimicry in plants. Evol Biol 1978; 11:365 - 403
- Rothschild M. Gilbert LE, Raven PH. Remarks on carotenoids in the evolution of signals. Coevolution of animals and plants 1980; Austin, TX University of Texas Press 20 - 51
- Rothschild M. The red smell of danger. New Sci 1986; September 4 111:34 - 36
- Harborne JB. Introduction to ecological biochemistry 1982; London, UK Academic Press
- Williamson GB. Plant mimicry: evolutionary constraints. Biol J Linn Soc 1982; 18:49 - 58
- Hill ME. The effect of aposematic coloration on the food preference of Aphelocoma coerulescens, the Florida scrub jay. Bios 2006; 77:97 - 106
- Karageorgou P, Buschmann C, Manetas Y. Red leaf color as a warning signal against insect herbivory: Honest or mimetic?. Flora 2008; 203:648 - 652
- Archetti M. Classification of hypotheses for the evolution of autumn colours. Oikos 2009; 118:328 - 333
- Herrera CM. Aposematic insects as six-legged fruits: incidental short-circuiting of their defense by frugivorous birds. Am Nat 1985; 126:286 - 293
- Gamberale-Stille G, Tullberg BS. Fruit or aposematic insect? Context-dependent colour preferences in domestic chicks. Proc Roy Soc Lond B 2001; 268:2525 - 2529
- Lev-Yadun S, Inbar M. Defensive ant, aphid and caterpillar mimicry in plants. Biol J Linn Soc 2002; 77:393 - 398
- Herrera J. The reproductive biology of a riparian Mediterranean shrub, Nerium oleander L. (Apocynaceae). Bot J Linn Soc 1991; 106:147 - 172
- Langford SD, Boor PJ. Oleander toxicity: an examination of human and animal toxic exposures. Toxicology 1996; 109:1 - 13
- Osterloh J, Herold S, Pond S. Oleander interference in the digoxin radioimmunoassay in a fatal ingestion. JAMA 1982; 247:1596 - 1597
- Pearn J. Covacevich J, Davie P, Pearn J. Oleander poisoning. Toxic plants & animals; a guide for Australia 1987; Brisbane, Australia Queensland Museum 37 - 49
- Tsahar E, Friedman J, Izhaki I. Impact on fruit removal and seed predation of a secondary metabolite, emodin, in Rhamnus alaternus fruit pulp. Oikos 2002; 99:290 - 299
- Izhaki I. Seed dispersal by birds in east Mediterranean scrub 1986; Jerusalem, Israel The Hebrew University of Jerusalem Ph.D. dissertation. (in Hebrew with English summary).
- Herrera CM. A study of avian frugivores, bird-dispersed plants, and their interaction in Mediterranean scrublands. Ecol Monogr 1984; 54:1 - 23
- Jordano P. Pre-dispersal biology of Pistacia lentiscus (Anacardiaceae): cumulative effects on seed removal by birds. Oikos 1989; 55:375 - 386
- Traveset A. Cumulative effects on the reproductive output of Pistacia terebinthus (Anacardiaceae). Oikos 1994; 71:152 - 162
- Jordano P. Migrant birds are the main seed dispersers of blackberries in southern Spain. Oikos 1982; 38:183 - 193
- Jordano P. Seed weight variation and differential avian dispersal in blackberries Rubus ulmifolius. Oikos 1984; 43:149 - 153
- Zohary M. Vegetal landscapes of Israel 1980; Tel Aviv, Israel Am Oved (in Hebrew).
- Feinbrun-Dothan N, Danin A. Analytical flora of Eretz-Israel 1991; Jerusalem, Israel Cana Publishing House Ltd (in Hebrew).
- Schaefer HM, Levey DJ, Schaefer V, Avery ML. The role of chromatic and achromatic signals for fruit detection by birds. Behav Ecol 2006; 17:784 - 789
- Rothschild M, von Euw L, Reichstein T. Cardiac glycosides in the Oleander aphid Aphis nerii. J Insect Physiol 1970; 16:1141 - 1145
- Sherburne JA. Effects of seasonal changes in the abundance and chemistry of the fleshy fruits of northeastern woody shrubs on patterns of exploitation by frugivorous birds 1972; Cornell University Ph.D. Thesis
- Maw MG. Rhamnus cathartica L., common or European buckthorn (Rhamnaceae). Biological control program against insects and weeds in Canada 1969–1980 1981; London, UK Commonwealth Agricultural Bureau 185 - 189
- Izhaki I. Emodin - a secondary metabolite with multiple ecological functions in higher plants. New Phytol 2002; 155:205 - 217
- Bas JM, Gómez C, Pons P. Fruit production and pre-dispersal seed fall and predation in Rhamnus alaternus (Rhamnaceae). Acta Oecol 2005; 27:115 - 123
- Irwin RE, Strauss SY. The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 2003; 84:1733 - 1743
- Strauss SY, Irwin RE. Optimal defence theory and flower petal colour predict variation in the secondary chemistry of wild radish. J Ecol 2004; 92:132 - 141
- Lev-Yadun S, Ne'eman G, Shanas U. A sheep in wolf's clothing: Do carrion and dung odours of flowers not only attract pollinators but also deter herbivores?. BioEssays 2009; 31:84 - 88
- Dobson HEM, Bergström G. The ecology and evolution of pollen odors. Plant Syst Evol 2000; 222:63 - 87
- Gronquist M, Bezzerides A, Attygalle A, Meinwald J, Eisner M, Eisner T. Attractive and defensive functions of the ultraviolet pigments of a flower (Hypericum calycinum). Proc Natl Acad Sci USA 2001; 98:13745 - 13750
- Herrera CM, Medrano M, Rey PJ, Sánchez-Lafuente AM, Garcia MB, Guitián J, et al. Interaction of pollinators and herbivores on plant fitness suggests a pathway for correlated evolution of mutualism- and antagonism-related traits. Proc Natl Acad Sci USA 2002; 99:16823 - 16828
- Stephenson AG. Toxic nectar deters nectar thieves of Catalpa speciosa. Am Midl Nat 1981; 105:381 - 383
- Johnson SD, Hargreaves AL, Brown M. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology 2006; 87:2709 - 2716
- Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. Diversity and distribution of floral scent. Bot Rev 2006; 72:1 - 120
- Hansen DM, Olesen JM, Mione T, Johnson SD, Müller CB. Coloured nectar: Distribution, ecology, and evolution of an enigmatic floral trait. Biol Rev 2007; 82:83 - 111
- Janzen DH. Rosenthal GA, Janzen DH. New horizons in the biology of plant defenses. Herbivores their interaction with secondary plant metabolites 1979; Orlando, FL Academic Press 331 - 350
- Struempf HM, Schondube JE, del Rio CM. The cyanogenic glycoside amygdalin does not deter consumption of ripe fruit by cedar waxwings. Auk 1999; 116:749 - 758
- Abeles FB, Morgan PW, Saltveit ME Jr. Ethylene in plant biology 1992; 2nd ed. San Diego, CA Academic Press
- Janzen DH. Why fruits rot, seeds mold, and meat spoils. Am Nat 1977; 111:691 - 713
- Herrera CM. Defense of ripe fruit from pests: Its significance in relation to plant-disperser interactions. Am Nat 1982; 120:218 - 241
- Manzur MI, Courtney SP. Influence of insect damage in fruits of hawthorn on bird foraging and seed dispersal. Oikos 1984; 43:265 - 270
- Borowicz VA. Do vertebrates reject decaying fruit? An experimental test with Cornus amomum fruits. Oikos 1988; 53:74 - 78
- Buchholz R, Levey DJ. The evolutionary triad of microbes, fruits, and seed dispersers: an experiment in fruit choice by cedar waxwings, Bombycilla cedrorum. Oikos 1990; 59:200 - 204
- Cipollini ML, Stiles EW. Fruit rot, antifungal defense, and palatability of fleshy fruits for frugivorous birds. Ecology 1993; 74:751 - 762
- Marchand D, McNeil JN. Avoidance of intraspecific competition via host modification in a grazing, fruit-eating insect. Anim Behav 2004; 67:397 - 402
- Gould KS, Lee DW, Callow JA. Anthocyanins in leaves. Adv Bot Res 2002; 37
- Close DC, Beadle CL. The ecophysiology of foliar anthocyanin. Bot Rev 2003; 69:149 - 161
- Gould KS. Nature's Swiss army knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 2004; 5:314 - 320
- Lev-Yadun S, Dafni A, Inbar M, Izhaki I, Ne`eman G. Colour patterns in vegetative parts of plants deserve more research attention. Trends Plant Sci 2002; 7:59 - 60
- Lev-Yadun S, Dafni A, Flaishman MA, Inbar M, Izhaki I, Katzir G, et al. Plant coloration undermines herbivorous insect camouflage. BioEssays 2004; 26:1126 - 1130