Abstract
Redox signals play important roles in many developmental and metabolic processes, in particular in chloroplasts and mitochondria. Furthermore, redox reactions are crucial for protein folding via the formation of inter- or intramolecular disulfide bridges. Recently, redox signals were described to be additionally involved in regulation of protein import: in mitochondria, a disulfide relay system mediates retention of cystein-rich proteins in the intermembrane space by oxidizing them. Two essential proteins, the redox-activated receptor Mia40 and the sulfhydryl oxidase Erv1 participate in this pathway. In chloroplasts, it becomes apparent that protein import is affected by redox signals on both the outer and inner envelope: at the level of the Toc complex (translocon at the outer envelope of chloroplasts), the formation/reduction of disulfide bridges between the Toc components has a strong influence on import yield. Moreover, the stromal metabolic redox state seems to be sensed by the Tic complex (translocon at the inner envelope of chloroplasts) that is able to adjust translocation efficiency of a subgroup of redox-related preproteins accordingly. This review summarizes the current knowledge of these redox-regulatory pathways and focuses on similarities and differences between chloroplasts and mitochondria.
Introduction
It is a widely accepted theory that both mitochondria and chloroplasts derived from an endosymbiotic event,Citation1 and in many of their biochemical and physiological properties they still resemble their bacterial ancestors. During evolution, the endosymbionts were transformed into semi-autonomous organelles mainly by transferring most of their genes to the host cell nucleus.Citation2 Thereby, chloroplasts and mitochondria became dependent on post-translational, energy-dependent protein import from the surrounding cell. Not surprisingly, protein targeting to and import into both organelles have much in common:Citation3–Citation6 for example, most chloroplastic and mitochondrial preproteins are synthesized on cytosolic ribosomes with amino-terminal extensions, which can be recognized by specific receptors on the surface of the organelles. Moreover, the preproteins are transported into the organelles with the help of membrane-embedded translocation complexes located in both organelle membranes, which share many features. Upon arrival in the stroma or matrix, respectively, the aminoterminal extensions are proteolytically removed and the mature proteins are folded into their final conformations, a process that is assisted by chaperones.
Although the structures and functions of the components that constitute the translocation machineries have been described in great detail during the last years, our understanding of the regulation of import into both organelles is in its infancy. However, recent studies suggest that the pathways by which regulation is achieved seem to share some similarities as well, since both chloroplasts and mitochondria were found to use redox signals for the regulation of translocation. This is of great interest, as redox signals play important roles in both endosymbionts, which are the main producers of redox energy in the cell (mediated by the photosynthetic electron transport chain or the respiratory electron transport chain, respectively). In addition to delivering energy, electron transfer processes are used in a regulatory manner, and redox signals are known to be fundamental for many metabolic and developmental processes in both organelles. Redox regulation is often mediated by thioredoxins (Trx m, f, y and x in chloroplasts, Trx h and o in mitochondria) which are able to (de-) activate enzymes by reversible reduction of disulfide bonds.Citation7–Citation9 Additionally, Trx-like proteins are known to mediate oxidative folding of substrate proteins in the bacterial periplasm and the endoplasmic reticulum of eukaryotic cells.Citation10–Citation12 The resulting disulfide bonds can have a purely structural function as in protein folding, which is in contrast to the regulatory role in Trx-dependent enzyme (de-)activation. Nevertheless, the function of many proteins depends also on the correct assembly of structural disulfide bridges.
In this review, we briefly summarize the current knowledge of import redox-regulation in both chloroplasts and mitochondria, and take a closer look at similarities and differences of these processes: for mitochondria, a thiol oxidizing machinery located in the inter membrane space (IMS) was described that is necessary for the retention of small IMS proteins.Citation13–Citation15 Protein import into chloroplasts is also affected by a thiol-mediated regulation, but additionally the metabolic redox state was found to influence the import yield.Citation16
Redox-Regulation of Protein Import into Mitochondria: Import of Small IMS Proteins
Thiol oxidation in non-plant eukaryotic cells was recently discovered to occur not only in the endoplasmic reticulum but also in the IMS of mitochondria.Citation13 In this compartment, a number of proteins can be found that do not possess a cleavable presequence, but that contain conserved cysteine residues, preferentially arranged in CX3C or CX9C motifs. Members of this group are e.g., Cox12, Cox17, Sod1 and the small Tim proteins. Citation17,Citation18 The import of these proteins was found to be dependent on the Tom (translocon of the outer mitochondrial membrane), but not on the Tim (translocon of the inner mitochondrial membrane) complex. Additionally, a thiol oxidizing machinery located in the IMS was described that is required for correct import of these small IMS proteins.Citation13–Citation15,Citation19 Two IMS components were found to be essential for this so-called “disulfide relay system” (): the sulfhydryl oxidase Erv1Citation20,Citation21 and the redox-activated protein receptor Mia40.Citation22–Citation25 In this import pathway, proteins are first translocated into the IMS with the help of the Tom complex in a reduced, unfolded and import-competent state. Subsequently, Mia40 (that contains six conserved cysteine residues itself) directly interacts with these incoming target proteins via their CX3C or CX9C motifs leading to the formation of (one or two) intermolecular disulfide bridges. Finally, these mixed disulfides are transferred to the preproteins which are thus released from Mia40 in an oxidized and folded conformation. As Mia40 needs to be in an oxidized state to fulfil its function, re-oxidation is necessary and is mediated by Erv1. Reduced Erv1 itself donates the electrons to molecular oxygen (via cytochrome and the respiratory chain) in order to become re-oxidized. Recently, another component was identified that also participates in the import of cysteine-rich IMS proteins: the zinc-binding protein Hot13.Citation26 This protein was initially described as important factor for the assembly of small Tim proteins in the IMS,Citation27 but it was additionally shown to interact with Mia40. Thus, Mia40 is maintained in a zinc-free state, which makes re-oxidation by Erv1 more efficient.
The importance of this oxidative pathway for small IMS proteins is also reflected by the fact that both Mia40 and Erv1 are essential proteins in yeast.Citation22,Citation28 This redox-driven protein import is necessary, since the small IMS proteins are able to back across the Tom channel to the cytosol in their un-oxidized, import-competent conformation. In the oxidized state, however, the proteins are unable to pass through the Tom pore and are thus “trapped” in the IMS. Thus, protein folding in the IMS can be regarded as the “driving force” of the described import pathway.
Redox-Regulation of Protein Import into Chloroplasts: Two Very Different Ways
Over the last two decades, several observations have led to the hypothesis that protein import into chloroplasts might be regulated by redox signals.Citation29–Citation33 But only recently, these implications have been tested in a more elaborate approach, demonstrating that import is indeed regulated in a redox-dependent manner.Citation16 Interestingly, not only one, but several regulatory pathways could be distinguished that are dependent on different redox signals: on the one hand, protein import was found to be stimulated by the reduction of disulfide bridges at the surface of the chloroplast (). This process affects subunits in the Toc complex (translocon at the outer envelope of chloroplasts) that all contain a number of conserved cysteine residues, and it was shown that the Toc channel, Toc75, and the Toc receptor components Toc159, Toc34 and Toc64 are involved in this redox regulation. The reduction of disulfide bridges was demonstrated to clearly increase protein import, whereas oxidation of thiols efficiently blocks translocation.Citation16,Citation29,Citation30 However, not all Toc proteins seem to work together to mediate regulation: the study suggests that intermolecular disulfide bridges are either generated between Toc75, Toc34 and Toc159 that subsequently form a hetero-oligomer, or between several Toc64 molecules that probably form homo-oligomers upon oxidation via intramolecular disulfide bonds. Two mechanisms of redox regulation by the formation of disulfide bridges have been proposed: firstly, the oxidized Toc159/Toc34/Toc75 oligomer might simply block the translocation channel for incoming preproteins. Alternatively, as all Toc receptors are targets of redox regulation, it seems likely that already the binding of preproteins to the receptor components is affected. Thus, the reduction of disulfides, which was shown to have a stimulating effect on protein import, might cause a higher flexibility of the Toc receptors, which might be necessary for efficient preprotein binding and subsequent handing over of the precursor to the channel protein Toc75.Citation34,Citation35
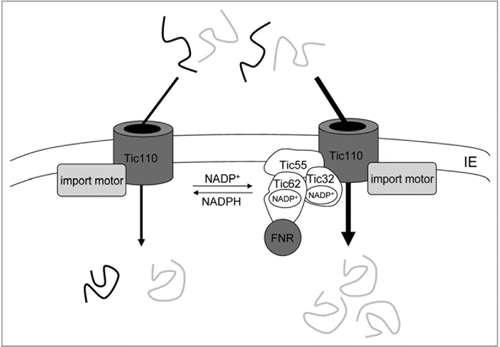
Another redox-regulatory pathway observed for import into chloroplasts is mediated by the stromal NADP+/NADPH ratio.Citation16 It was demonstrated that the generation of stromal NADP+ increases the import efficiency interestingly of not all, but a large number of preproteins. By contrast, a change in the NADP+/NADPH equlibrium towards a more reduced state (high NADPH amounts) did not enhance import, but either had no effect or even slightly inhibited protein translocation. The preprotein group that was found to be responsive to the NADP+/NADPH ratio included many photosynthetic proteins (e.g., ferredoxin-NADP+-oxidoreductase, FNR; glyceraldehyde 3-phosphate dehydrogenase, GAPDH; the small subunit of ribulose-1,5-bis-phosphate-carboxylase/-oxygenase, SSU) as well as key metabolic enzymes (e.g., malate dehydrogenase, MDH; glucose-6-phosphate dehydrogenase, G6PDH). The second, much smaller group of proteins that imported independently of changes in the stromal redox state included non-photosynthetic “house-keeping” proteins like nucleoside diphosphate kinase (NDPK) that are needed in constant amounts in the organelle and thus do not require import according to the chloroplast redox state. However, it would be too simple to distinguish only between import pathways of photosynthetic and non-photosynthetic proteins, as some photosynthetic enzymes were also found to import in a NADP+-independent manner (e.g., fructose-1,6-bisphosphatase, FBPase; phosphoglycerate kinase, PGK). It was thus proposed that not only the type of preprotein, but also the current metabolic state of the chloroplast determines the import pathway, as it is conceivable that the import of proteins like FBPase or PGK becomes dependent on the NADP+/NADPH ratio under certain yet unknown conditions. Still, the reduction equivalents seem to provide a signal transmitting information about the protein demand of the organelle to the translocation machinery. Changes in the metabolic NADP+/NADPH ratio were proposed to be sensed by the “redox regulon” of the Tic complex (translocon at the inner envelope of chloroplasts), which consists of the redox-active components Tic32, Tic55 and Tic62.Citation36 Interestingly, and important for this regulation pathway, both Tic62 and Tic32 contain functional NADP(H)-binding sites.Citation32,Citation37,Citation38 Additionally, both assemble dynamically with the Tic complex in a NADP+-dependent manner, Citation37 and Tic62 was furthermore described to interact strongly and specifically with FNR, a key photosynthetic enzyme.Citation32,Citation39 It is thought that these Tic proteins are recruited to the Tic “core” complex under certain conditions to mediate NADP+-dependent import regulation (). Consequently, different types of Tic subcomplexes might exist in the chloroplast, either comprised of only the channel Tic110 associated with stromal chaperones, or additionally including the Tic “redox regulon”. Thus, this regulatory pathway might provide a link between the redox state of the chloroplast and import of a number of redox-related proteins.
Similarities and Differences of Import Regulation into Chloroplasts and Mitochondria
At first glance, redox-regulation of import seems to share some similarities between chloroplasts and mitochondria: in both cases, the formation/reduction of disulfide bridges is involved. However, the mechanisms by which the oxidation of thiols leads to changes in the import yield differ significantly. In mitochondria, disulfide bridges are formed in the target preproteins to oxidize and thus fold them. This leads to a trapping of these proteins in the IMS, as they are now unable to slide back through the Tom channel into the cytosol. In chloroplasts on the other hand, the oxidation of thiol groups takes place in the channel and/or receptor components of the Toc complex thereby causing a blocking of the translocation channel. It remains to be tested whether mixed disulfides are additionally formed between the Toc components and incoming preproteins (as it is the case for Mia40) but there have not been any indications for this. Furthermore, these two thiol-dependent import pathways affect completely different precursor subgroups: in mitochondria, only small IMS proteins that contain conserved cysteine residues are dependent on the disulfide relay system mediated by Mia40 and Erv1 for their import. In chloroplasts, however, all proteins that use the general import pathway (and thus the Toc complex) for translocation are sensitive to the formation/reduction of disulfide bridges in the Toc components. Additionally, a second redox-regulatory system has been implemented in chloroplasts using the reduction equivalents NADP(H) as signals for the redox state of the organelle. A similar system is completely unknown in mitochondria so far. By this pathway, protein import can be adapted to the photosynthetic activity and thus the metabolic redox state. This can be considered as a “real” regulation, as the environmental or developmental conditions have the possibility to influence protein import yield, in contrast to the thiol-mediated pathways, where purely structural changes in the corresponding proteins lead to efficient import. Hence, redox-regulation of protein translocation into chloroplasts seems to be even more complex than in mitochondria, as it can be adjusted to the requirements of the organelle at any given time. Hence, both endosymbiotic organelles developed the use of redox-signals to regulate the import of at least subgroups of preproteins, although in very different ways.
More Similarities: Is there an Erv1 Homologue in Chloroplasts?
Interestingly, in a screen for further Trx targets in the chloroplast envelope, a recent study identified an Erv1 homologue in the outer plastid envelope fraction of barley chloroplasts using Trx affinity columns.Citation40 In this analysis, the Erv1 homologue eluted as one of the most prominent proteins after incubation of the Trx matrix with solubilized outer envelope. As further characterization of this protein was not the focus of this study, the role of this chloroplast Erv1 homologue needs to be established. However, because of its similarity to Erv1, a protein that is clearly implicated in disulfide bridge formation in mitochondria (see above) and also in bacteria,Citation41,Citation42 participation of the Erv1 homologue in redox-regulation of protein import into chloroplasts may be feasible. As the protein was identified in the outer envelope, one possibility would be an involvement in the regulation pathway by disulfide bridge formation in the Toc components as described above, maybe by oxidizing them (similar to its role in the Mia40 pathway) and thus controlling the import yield under conditions when the protein demand of the chloroplast is low. Alternatively, the Erv1 homologue could participate in a hypothetical import pathway of chloroplast IMS proteins. Although the function of this protein is completely unknown till now, its existence suggests that there are maybe more similarities between import regulation into chloroplasts and mitochondria than identified so far.
Figures and Tables
Figure 1 Redox-regulated protein import into mitochondria: the disulfide relay system. Simplified model of the components and steps involved in import of small IMS proteins. In the first step (1) the IMS proteins that contain conserved cysteine residues, are translocated into the IMS via the Tom complex. For high import efficiency, they have to be in a reduced, unfolded state. Upon arrival in the IMS, these proteins are oxidized and thus folded by the redox-activated import receptor Mia40 (2). Due to the folding, the IMS proteins are trapped in the IMS and cannot traverse back through the Tom channel into the cytosol. Subsequently, Mia40 directly interacts with the sulfhydryl oxidase Erv1 to get re-oxidized (3). Erv1 itself maintains in an oxidized state by the use of molecular oxygen as a final electron acceptor (4). Reduced and oxidized thiol groups are indicated by SH and SS, respectively.
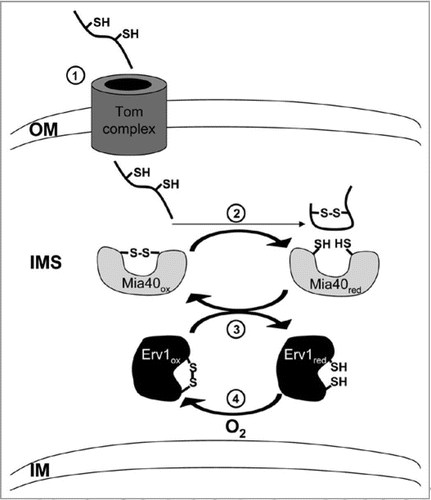
Figure 2 Redox-regulation of protein import into chloroplasts at the level of the Toc complex. The import yield of preproteins is strongly dependent on the formation or reduction of disulfide bridges in the Toc channel Toc75 and/or in the Toc receptors Toc159, Toc34 and Toc64. Reduction of disulfide groups lead to an increase in import efficiency (left part), whereas oxidation of thiol groups inhibits preprotein import (right part). Reduction of the active disulfide groups seems to allow greater flexibility of the Toc receptors which is likely necessary for efficient preprotein binding and subsequent transfer of the precursor to the Toc75 channel protein. Blocking of import on the other hand seems to be achieved by the formation of intermolecular disulfide bridges between Toc159/Toc75/Toc34, leading to the formation of a heterotrimer, (indicated by the close proximity of the components in the left part) and/or by homo-oligomerization of Toc64. All preproteins using the Toc complex seem to be influenced by this thiol-mediated regulation. Reduced and oxidized thiol groups are indicated by SH and SS, respectively.
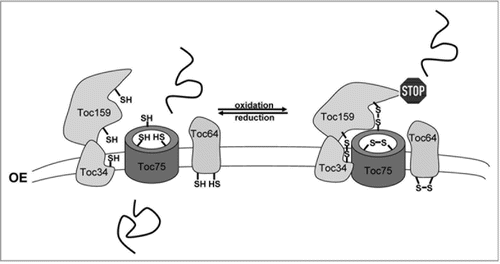
Figure 3 Influence of the stromal redox state on import regulation at the level of the Tic translocon. Redox-regulation is achieved by changes in the metabolic NADP+/NADPH ratio: for a subgroup of mostly redox-related preproteins (grey), high levels of NADP+ were found to increase import yield (right part, shown by thick arrow). Sensing of high NADP+ concentrations seems to be mediated by the “redox regulon” of the Tic complex, consisting of Tic32, Tic55 and Tic62 (associated with ferredoxin NADP+-oxidoreductase, FNR). According to the current view, this regulon is recruited to the Tic “core” complex, consisting of at least Tic110 as central translocation channel and associated chaperones (indicated as import motor) under conditions where the chloroplast has a high demand for redox-related proteins (high NADP+ levels). By contrast, a second subtype of Tic translocons is proposed that only contains Tic110 as channel with the associated motor complex (left part). This latter complex provides basic redox-independent import for all proteins, including the redox-related ones (grey) but also for “house-keeping” proteins (black). Thus, the Tic redox regulon may sense photosynthesisderived redox signals and modulate protein import according to the metabolic requirements of the organelle through its dynamic association with Tic110.
References
- Margulis L. Origin of eukaryotic cells 1970; New Haven CT, USA Yale University Press
- Leister D. Origin, evolution and genetic effects of nuclear insertions of organelle DNA. Trends Genet 2005; 21:655 - 663
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem 2007; 76:723 - 749
- Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner N. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep 2008; 9:42 - 49
- Jarvis P. Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol 2008; 179:257 - 285
- Stengel A, Soll J, Bolter B. Protein import into chloroplasts: new aspects of a well-known topic. Biol Chem 2007; 388:765 - 772
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annu Rev Plant Biol 2005; 56:187 - 220
- Hisabori T, Motohashi K, Hosoya-Matsuda N, Ueoka-Nakanishi H, Romano PG. Towards a functional dissection of thioredoxin networks in plant cells. Photochem Photobiol 2007; 83:145 - 151
- Montrichard F, Alkhalfioui F, Yano H, Vensel WH, Hurkman WJ, Buchanan BB. Thioredoxin targets in plants: The first 30 years. J Proteomics 2009; 72:452 - 474
- McCarthy AA, Haebel PW, Torronen A, Rybin V, Baker EN, Metcalf P. Crystal structure of the protein disulfide bond isomerase, DsbC, from Escherichia coli. Nat Struct Biol 2000; 7:169 - 196
- Bardwell JC, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell 1991; 67:581 - 589
- Zapun A, Bardwell JC, Creighton TE. The reactive and destabilizing disulfide bond of DsbA, a protein required for protein disulfide bond formation in vivo. Biochem 1993; 32:5083 - 5092
- Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K, et al. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 2005; 121:1059 - 1069
- Rissler M, Wiedemann N, Pfannschmidt S, Gabriel K, Guiard B, Pfanner N, et al. The essential mitochondrial protein Erv1 cooperates with Mia40 in biogenesis of intermembrane space proteins. J Mol Biol 2005; 353:485 - 492
- Tokatlidis K. A disulfide relay system in mitochondria. Cell 2005; 121:965 - 967
- Stengel A, Benz P, Buchanan B, Soll J, Bölter B. Preprotein import into chloroplasts via the Toc and Tic complexes is regulated by redox signals in Pisum sativum. Mol Plant 2009; doi:10.11093/mp/ssp043
- Curran SP, Leuenberger D, Oppliger W, Koehler CM. The Tim9p-Tim10p complex binds to the transmembrane domains of the ADP/ATP carrier. EMBO J 2002; 21:942 - 953
- Herrmann JM, Kauff F, Neuhaus HE. Thiol oxidation in bacteria, mitochondria and chloroplasts: common principles but three unrelated machineries?. Biochim Biophys Acta 2009; 1793:71 - 77
- Herrmann JM, Köhl R. Catch me if you can! Oxidative protein trapping in the intermembrane space of mitochondria. J Cell Biol 2007; 176:559 - 563
- Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K. Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol 2005; 353:937 - 944
- Bihlmaier K, Mesecke N, Terzyiska N, Bien M, Hell K, Herrmann JM. The disulfide relay system of mitochondria is connected to the respiratory chain. J Cell Biol 2007; 179:389 - 395
- Chacinska A, Pfannschmidt S, Wiedemann N, Kozjak V, Sanjuan Szklarz LK, Schulze-Specking A, et al. Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J 2004; 23:3735 - 3746
- Terziyska N, Lutz T, Kozany C, Mokranjac D, Mesecke N, Neupert W, et al. Mia40, a novel factor for protein import into the intermembrane space of mitochondria is able to bind metal ions. FEBS Lett 2005; 579:179 - 184
- Hofmann S, Rothbauer U, Muhlenbein N, Baiker K, Hell K, Bauer MF. Functional and mutational characterization of human MIA40 acting during import into the mitochondrial intermembrane space. J Mol Biol 2005; 353:517 - 528
- Grumbt B, Stroobant V, Terziyska N, Israel L, Hell K. Functional characterization of Mia40p, the central component of the disulfide relay system of the mitochondrial intermembrane space. J Biol Chem 2007; 282:37461 - 37470
- Mesecke N, Bihlmaier K, Grumbt B, Longen S, Terziyska N, Hell K, et al. The zinc-binding protein Hot13 promotes oxidation of the mitochondrial import receptor Mia40. EMBO Rep 2008; 9:1107 - 1113
- Curran SP, Leuenberger D, Leverich EP, Hwang DK, Beverly KN, Koehler CM. The role of Hot13p and redox chemistry in the mitochondrial TIM22 import pathway. J Biol Chem 2008; 279:43744 - 43751
- Lange H, Lisowsky T, Gerber J, Muhlenhoff U, Kispal G, Lill R. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep 2001; 2:715 - 720
- Seedorf M, Soll J. Copper chloride, an inhibitor of protein import into chloroplasts. FEBS Lett 1995; 367:19 - 22
- Pilon R, de Kruijff B, Weisbeek PJ. New insights into the import mechanism of the ferredoxin precursor into chloroplasts. J Biol Chem 1992; 267:2548 - 2556
- Frydman J, Hohfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci 1997; 22:87 - 92
- Stengel A, Benz P, Balsera M, Soll J, Bölter B. Tic62-Redox-regulated translocon composition and dynamics. J Biol Chem 2008; 283:6656 - 6667
- Hirohashi T, Hase T, Nakai M. Maize non-photosynthetic ferredoxin precursor is mis-sorted to the intermembrane space of chloroplasts in the presence of light. Plant Physiol 2001; 125:2154 - 2163
- Becker T, Jelic M, Vojta A, Radunz A, Soll J, Schleiff E. Preprotein recognition by the Toc complex. EMBO J 2004; 23:520 - 530
- Schleiff E, Jelic M, Soll J. A GTP-driven motor moves proteins across the outer envelope of chloroplasts. Proc Natl Acad Sci USA 2003; 100:4604 - 4609
- Benz P, Soll J, Bölter B. Chloroplast protein import—The Tic complex: composition, function and regulation. FEBS J 2008; 276:1166 - 1176
- Chigri F, Hölmann F, Stamp A, Stammers DK, Bölter B, Soll J, et al. Calcium regulation of chloroplast protein translocation is mediated by calmodulin binding to Tic32. Proc Natl Acad Sci USA 2006; 103:16051 - 16056
- Hörmann F, Küchler M, Sveshnikov D, Oppermann U, Li Y, Soll J. Tic32, an essential component in chloroplast biogenesis. J Biol Chem 2004; 279:34756 - 34762
- Küchler M, Decker S, Hörmann F, Soll J, Heins L. Protein import into chloroplasts involves redox-regulated proteins. EMBO J 2002; 21:6136 - 6145
- Bartsch S, Monnet J, Selbach K, Quigley F, Gray J, von Wettstein D, et al. Three thioredoxin targets in the inner envelope membrane of chloroplasts function in protein import and chlorophyll metabolism. Proc Natl Acad Sci USA 2008; 105:4933 - 4938
- Lee J, Hofhaus G, Lisowsky T. Erv1p from Saccharomyces cerevisiae is a FADlinked sulfhydryl oxidase. FEBS Lett 2000; 477:62 - 66
- Gerber J, Muhlkenhoff U, Hofhaus G, Lill R, Lisowsky T. Yeast ERV2p is the first microsomal FAD-linked sulhydryl oxidase of the Erv1p/Alrp protein family. J Biol Chem 2001; 276:23486 - 234891